Feasibility and User Experience of Digital Patient Monitoring for Real-World Patients With Lung or Breast Cancer
- PMID: 38007400
- PMCID: PMC10994260
- DOI: 10.1093/oncolo/oyad289
Feasibility and User Experience of Digital Patient Monitoring for Real-World Patients With Lung or Breast Cancer
Abstract
Background: Digital patient monitoring (DPM) tools can facilitate early symptom management for patients with cancer through systematic symptom reporting; however, low adherence can be a challenge. We assessed patient/healthcare professional (HCP) use of DPM in routine clinical practice.
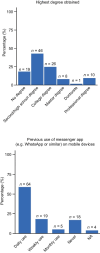
Materials and methods: Patients with locally advanced/metastatic lung cancer or HER2-positive breast cancer received locally approved/reimbursed drugs alongside DPM, with elements tailored by F. Hoffmann-La Roche Ltd, on the Kaiku Health DPM platform. Patient access to the DPM tool was through their own devices (eg, laptops, PCs, smartphones, or tablets), via either a browser or an app on Apple iOS or Android devices. Coprimary endpoints were patient DPM tool adoption (positive threshold: 60%) and week 1-6 adherence to weekly symptom reporting (positive threshold: 70%). Secondary endpoints included experience and clinical impact.
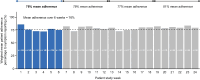
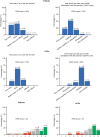
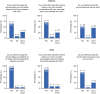
Results: At data cutoff (June 9, 2022), adoption was 85% and adherence was 76%. Customer satisfaction and effort scores for patients were 76% and 82%, respectively, and 83% and 79% for HCPs. Patients spent approximately 10 minutes using the DPM tool and completed approximately 1.0 symptom questionnaires per week (completion time 1-4 minutes). HCPs spent approximately 1-3 minutes a week using the tool per patient. Median time to HCP review for alerted versus non-alerted symptom questionnaires was 19.6 versus 21.5 hours. Most patients and HCPs felt that the DPM tool covered/mostly covered symptoms experienced (71% and 75%), was educational (65% and 92%), and improved patient-HCP conversations (70% and 83%) and cancer care (51% and 71%).
Conclusion: The DPM tool demonstrated positive adoption, adherence, and user experience for patients with lung/breast cancer, suggesting that DPM tools may benefit clinical cancer care.
Keywords: breast cancer; digital patient monitoring; eHealth; lung cancer; patient-reported outcomes; symptom monitoring.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
All authors received research support in the form of third-party medical writing assistance from F. Hoffmann-La Roche Ltd. Edurne Arriola reported consulting/advisory relationships with Lilly and Boehringer Ingelheim, and honoraria from Roche, MSD, BMS, AstraZeneca, Pfizer, Thermo Fisher Scientific, Guardant Health, and Takeda. Jana Jaal reported research funding from Roche, and consulting/advisory relationships with MSD and AstraZeneca. Anne Edvardsen reported research funding from Roche. Maria Silvoniemi reported a consulting/advisory relationship with Roche. Antonio Araujo reported research funding from Roche. Anders Vikström reported consulting/advisory relationships with Merck, BMS, MSD, and Pfizer, and honoraria from MSD, AstraZeneca, Roche, and BMS. Eleni Zairi and Mari Carmen Rodriguez-Mues: Authors declare no other competing interests. Marco Roccato is an employee of Kaiku Health. Sophie Schneider is an employee of F. Hoffmann-La Roche AG, with ownership interests. Johannes Ammann is an employee of F. Hoffmann-La Roche AG, with ownership interests.
Figures
Similar articles
-
Assessing the impact of digital patient monitoring on health outcomes and healthcare resource usage in addition to the feasibility of its combination with at-home treatment, in participants receiving systemic anticancer treatment in clinical practice: protocol for an interventional, open-label, multicountry platform study (ORIGAMA).BMJ Open. 2023 Apr 19;13(4):e063242. doi: 10.1136/bmjopen-2022-063242. BMJ Open. 2023. PMID: 37076159 Free PMC article.
-
Digital Monitoring and Management of Patients With Advanced or Metastatic Non-Small Cell Lung Cancer Treated With Cancer Immunotherapy and Its Impact on Quality of Clinical Care: Interview and Survey Study Among Health Care Professionals and Patients.J Med Internet Res. 2020 Dec 21;22(12):e18655. doi: 10.2196/18655. J Med Internet Res. 2020. PMID: 33346738 Free PMC article.
-
A randomized trial of weekly symptom telemonitoring in advanced lung cancer.J Pain Symptom Manage. 2014 Jun;47(6):973-89. doi: 10.1016/j.jpainsymman.2013.07.013. Epub 2013 Nov 7. J Pain Symptom Manage. 2014. PMID: 24210705 Free PMC article. Clinical Trial.
-
Telephone interventions for symptom management in adults with cancer.Cochrane Database Syst Rev. 2020 Jun 2;6(6):CD007568. doi: 10.1002/14651858.CD007568.pub2. Cochrane Database Syst Rev. 2020. PMID: 32483832 Free PMC article.
-
Evaluation of patient reporting of adverse drug reactions to the UK 'Yellow Card Scheme': literature review, descriptive and qualitative analyses, and questionnaire surveys.Health Technol Assess. 2011 May;15(20):1-234, iii-iv. doi: 10.3310/hta15200. Health Technol Assess. 2011. PMID: 21545758 Review.
Cited by
-
Optimizing Adjuvant Care in Early Breast Cancer: Multidisciplinary Strategies and Innovative Models from Canadian Centers.Curr Oncol. 2025 Jul 14;32(7):402. doi: 10.3390/curroncol32070402. Curr Oncol. 2025. PMID: 40710212 Free PMC article. Review.
-
Systematic review on the technology's role in supporting lung cancer patients in the treatment journey.NPJ Digit Med. 2025 Aug 13;8(1):516. doi: 10.1038/s41746-025-01913-7. NPJ Digit Med. 2025. PMID: 40796947 Free PMC article.
-
Improving real-world evaluation of patient- and physician-reported tolerability: niraparib for recurrent ovarian cancer (NiQoLe).JNCI Cancer Spectr. 2025 Jan 3;9(1):pkae114. doi: 10.1093/jncics/pkae114. JNCI Cancer Spectr. 2025. PMID: 39673810 Free PMC article. Clinical Trial.
-
Patient-reported outcome measure (PROM) programs for monitoring symptoms among patients treated with immunotherapy: a scoping review.JNCI Cancer Spectr. 2024 Nov 1;8(6):pkae102. doi: 10.1093/jncics/pkae102. JNCI Cancer Spectr. 2024. PMID: 39468738 Free PMC article.
-
Real-world ePRO use and clinical outcomes using electronic patient-reported symptom monitoring for patients with advanced non-small-cell lung cancer receiving first-line pembrolizumab.J Comp Eff Res. 2025 Feb;14(2):e240122. doi: 10.57264/cer-2024-0122. Epub 2025 Jan 17. J Comp Eff Res. 2025. PMID: 39819032 Free PMC article.
References
-
- Brahmer JR, Lacchetti C, Schneider BJ, et al. . Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714-1768. 10.1200/JCO.2017.77.6385 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

