Imiquimod-induced pruritus in female wild-type and knockin Wistar rats: underscoring behavioral scratching in a rat model for antipruritic treatments
- PMID: 38007440
- PMCID: PMC10675923
- DOI: 10.1186/s13104-023-06627-1
Imiquimod-induced pruritus in female wild-type and knockin Wistar rats: underscoring behavioral scratching in a rat model for antipruritic treatments
Abstract
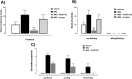
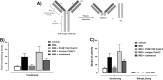
Objectives: Animal models of skin disease are used to evaluate therapeutics to alleviate disease. One common clinical dermatological complaint is pruritus (itch), but there is a lack of standardization in the characterization of pre-clinical models and scratching behavior, a key itch endpoint, is often neglected. One such model is the widely used imiquimod (IMQ) mouse model of psoriasis. However, it lacks characterized behavioral attributes like scratching, nor has widely expanded to other species like rats. Given these important attributes, this study was designed to broaden the characterization beyond the expected IMQ-induced psoriasis-like skin inflammatory skin changes and to validate the role of a potential therapeutic agent for pruritus in our genetic rat model. The study included female Wistar rats and genetically modified knockin (humanized proteinase-activated receptor 2 (F2RL1) female rats, with the widely used C57BL/6 J mice as a methodology control for typical IMQ dosing.
Results: We demonstrate that the IMQ model can be reproduced in rats, including their genetically modified derivatives, and how scratching can be used as a key behavioral endpoint. We systemically delivered an anti-PAR2 antibody (P24E1102) which reversed scratching bouts-validating this behavioral methodology and have shown its feasibility and value in identifying effective antipruritic drugs.
Keywords: Imiquimod; Pruritus; Psoriasis; Scratching bout; Wistar rat.
© 2023. The Author(s).
Conflict of interest statement
Teva Pharmaceuticals Industries Ltd. develops, produces and markets affordable, high quality generic drugs and specialty pharmaceuticals. Porsolt SAS is a contract research organization that conducted part of the study through a fee-for-service agreement with Teva Pharmaceuticals Industries Ltd. At the time of the study, PG, FS, KW were employed by Porsolt. KLW, DL, LW and JS were employed by Teva Pharmaceuticals Industries Ltd.
Figures

Similar articles
-
Thymol activates TRPM8-mediated Ca2+ influx for its antipruritic effects and alleviates inflammatory response in Imiquimod-induced mice.Toxicol Appl Pharmacol. 2020 Nov 15;407:115247. doi: 10.1016/j.taap.2020.115247. Epub 2020 Sep 22. Toxicol Appl Pharmacol. 2020. PMID: 32971067
-
Antipruritic Effects of Janus Kinase Inhibitor Tofacitinib in a Mouse Model of Psoriasis.Acta Derm Venereol. 2019 Mar 1;99(3):298-303. doi: 10.2340/00015555-3086. Acta Derm Venereol. 2019. PMID: 30460374
-
Mouse model of imiquimod-induced psoriatic itch.Pain. 2016 Nov;157(11):2536-2543. doi: 10.1097/j.pain.0000000000000674. Pain. 2016. PMID: 27437787 Free PMC article.
-
Anti-pruritic effect of isothiocyanates: Potential involvement of toll-like receptor 3 signaling.Pharmacol Res Perspect. 2022 Dec;10(6):e01038. doi: 10.1002/prp2.1038. Pharmacol Res Perspect. 2022. PMID: 36507603 Free PMC article. Review.
-
Methods for preclinical assessment of antipruritic agents and itch mechanisms independent of mast-cell histamine.Biol Pharm Bull. 2015;38(5):635-44. doi: 10.1248/bpb.b15-00090. Biol Pharm Bull. 2015. PMID: 25947907 Review.
Cited by
-
Evaluation of Clobetasol and Tacrolimus Treatments in an Imiquimod-Induced Psoriasis Rat Model.Int J Mol Sci. 2024 Aug 26;25(17):9254. doi: 10.3390/ijms25179254. Int J Mol Sci. 2024. PMID: 39273201 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

