Phylogenetic structure of body shape in a diverse inland ichthyofauna
- PMID: 38007528
- PMCID: PMC10676429
- DOI: 10.1038/s41598-023-48086-5
Phylogenetic structure of body shape in a diverse inland ichthyofauna
Abstract
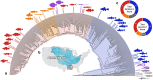
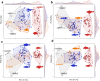
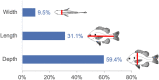
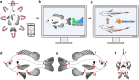
Body shape is a fundamental metric of animal diversity affecting critical behavioral and ecological dynamics and conservation status, yet previously available methods capture only a fraction of total body-shape variance. Here we use structure-from-motion (SFM) 3D photogrammetry to generate digital 3D models of adult fishes from the Lower Mississippi Basin, one of the most diverse temperate-zone freshwater faunas on Earth, and 3D geometric morphometrics to capture morphologically distinct shape variables, interpreting principal components as growth fields. The mean body shape in this fauna resembles plesiomorphic teleost fishes, and the major dimensions of body-shape disparity are similar to those of other fish faunas worldwide. Major patterns of body-shape disparity are structured by phylogeny, with nested clades occupying distinct portions of the morphospace, most of the morphospace occupied by multiple distinct clades, and one clade (Acanthomorpha) accounting for over half of the total body shape variance. In contrast to previous studies, variance in body depth (59.4%) structures overall body-shape disparity more than does length (31.1%), while width accounts for a non-trivial (9.5%) amount of the total body-shape disparity.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
How to tuna fish: constraint, convergence, and integration in the neurocranium of pelagiarian fishes.Evolution. 2023 Jun 1;77(6):1277-1288. doi: 10.1093/evolut/qpad056. Evolution. 2023. PMID: 36995728
-
The deep sea is a hot spot of fish body shape evolution.Ecol Lett. 2021 Sep;24(9):1788-1799. doi: 10.1111/ele.13785. Epub 2021 May 31. Ecol Lett. 2021. PMID: 34058793
-
The influence of size on body shape diversification across Indo-Pacific shore fishes.Evolution. 2019 Sep;73(9):1873-1884. doi: 10.1111/evo.13755. Epub 2019 May 24. Evolution. 2019. PMID: 31090919
-
Permian-Triassic Osteichthyes (bony fishes): diversity dynamics and body size evolution.Biol Rev Camb Philos Soc. 2016 Feb;91(1):106-47. doi: 10.1111/brv.12161. Epub 2014 Nov 27. Biol Rev Camb Philos Soc. 2016. PMID: 25431138 Review.
-
Phylogenetic perspectives on reef fish functional traits.Biol Rev Camb Philos Soc. 2018 Feb;93(1):131-151. doi: 10.1111/brv.12336. Epub 2017 May 2. Biol Rev Camb Philos Soc. 2018. PMID: 28464469 Review.
Cited by
-
An ecological trait matrix of Neotropical freshwater fishes.Sci Data. 2025 Jul 2;12(1):1127. doi: 10.1038/s41597-025-04674-w. Sci Data. 2025. PMID: 40603332 Free PMC article.
References
-
- Peters RH, Peters RH. The Ecological Implications Of Body Size. Cambridge Univ. Press; 1986.
-
- Raff RA. The Shape of Life: Genes, Development, and the Evolution of Animal Form. University of Chicago Press; 2012.
-
- Webb PW. Body form, locomotion and foraging in aquatic vertebrates. Am. Zool. 1984;24:107–120. doi: 10.1093/icb/24.1.107. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

