Biologically derived epicardial patch induces macrophage mediated pathophysiologic repair in chronically infarcted swine hearts
- PMID: 38007534
- PMCID: PMC10676365
- DOI: 10.1038/s42003-023-05564-w
Biologically derived epicardial patch induces macrophage mediated pathophysiologic repair in chronically infarcted swine hearts
Abstract
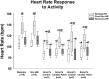
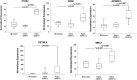
There are nearly 65 million people with chronic heart failure (CHF) globally, with no treatment directed at the pathologic cause of the disease, the loss of functioning cardiomyocytes. We have an allogeneic cardiac patch comprised of cardiomyocytes and human fibroblasts on a bioresorbable matrix. This patch increases blood flow to the damaged heart and improves left ventricular (LV) function in an immune competent rat model of ischemic CHF. After 6 months of treatment in an immune competent Yucatan mini swine ischemic CHF model, this patch restores LV contractility without constrictive physiology, partially reversing maladaptive LV and right ventricular remodeling, increases exercise tolerance, without inducing any cardiac arrhythmias or a change in myocardial oxygen consumption. Digital spatial profiling in mice with patch placement 3 weeks after a myocardial infarction shows that the patch induces a CD45pos immune cell response that results in an infiltration of dendritic cells and macrophages with high expression of macrophages polarization to the anti-inflammatory reparative M2 phenotype. Leveraging the host native immune system allows for the potential use of immunomodulatory therapies for treatment of chronic inflammatory diseases not limited to ischemic CHF.
© 2023. The Author(s).
Conflict of interest statement
Drs. Goldman, Koevary, Lancaster and Ms. Sherry Daugherty have disclosed a financial interest in Avery Therapeutics, Inc. to the University of Arizona. In addition, the University of Arizona has a financial interest in Avery Therapeutics, Inc. These interests have been reviewed and are being managed by the University of Arizona in accordance with its policies on outside interests. The work outlined in this report were the basis of forming a commercial entity, Avery Therapeutics. Drs. Goldman, Koevary, Lancaster and Ms. Sherry Daugherty have disclosed a financial interest in Avery Therapeutics, Inc. to the University of Arizona. In addition, the University of Arizona has a financial interest in Avery Therapeutics, Inc. These interests have been reviewed and are being managed by the University of Arizona in accordance with its policies on outside interests. All other authors declare no competing interests.
Figures







Similar articles
-
Tissue factor cytoplasmic domain exacerbates post-infarct left ventricular remodeling via orchestrating cardiac inflammation and angiogenesis.Theranostics. 2021 Sep 3;11(19):9243-9261. doi: 10.7150/thno.63354. eCollection 2021. Theranostics. 2021. PMID: 34646369 Free PMC article.
-
Infarcted Myocardium-Primed Dendritic Cells Improve Remodeling and Cardiac Function After Myocardial Infarction by Modulating the Regulatory T Cell and Macrophage Polarization.Circulation. 2017 Apr 11;135(15):1444-1457. doi: 10.1161/CIRCULATIONAHA.116.023106. Epub 2017 Feb 7. Circulation. 2017. PMID: 28174192
-
Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Patch in Rats With Heart Failure.Ann Thorac Surg. 2019 Oct;108(4):1169-1177. doi: 10.1016/j.athoracsur.2019.03.099. Epub 2019 May 7. Ann Thorac Surg. 2019. PMID: 31075250
-
Stem cells in cardiac repair.Future Cardiol. 2011 Jan;7(1):99-117. doi: 10.2217/fca.10.109. Future Cardiol. 2011. PMID: 21174514 Review.
-
Clinical aspects of left ventricular diastolic function assessed by Doppler echocardiography following acute myocardial infarction.Dan Med Bull. 2001 Nov;48(4):199-210. Dan Med Bull. 2001. PMID: 11767125 Review.
Cited by
-
Living Nanofiber-Enabled Cardiac Patches for Myocardial Injury.JACC Basic Transl Sci. 2025 Feb;10(2):227-240. doi: 10.1016/j.jacbts.2024.06.010. Epub 2024 Sep 4. JACC Basic Transl Sci. 2025. PMID: 40131159 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

