An immortal porcine preadipocyte cell strain for efficient production of cell-cultured fat
- PMID: 38007598
- PMCID: PMC10676435
- DOI: 10.1038/s42003-023-05583-7
An immortal porcine preadipocyte cell strain for efficient production of cell-cultured fat
Abstract
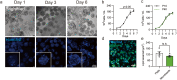
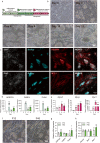
Adding adipose cells to cell-cultured meat can provide a distinctive aroma and juicy texture similar to real meat. However, a significant challenge still exists in obtaining seed cells that can be propagated for long periods, maintain their adipogenic potential, and reduce production costs. In this study, we present a cell strain derived from immortalized porcine preadipocytes that can be subculture for over 40 passages without losing differentiation capacity. This cell strain can be differentiated within 3D bioscaffolds to generate cell-cultured fat using fewer chemicals and less serum. Additionally, it can be expanded and differentiated on microcarriers with upscaled culture to reduce costs and labor. Moreover, it can co-differentiate with muscle precursor cells, producing a pattern similar to real meat. Therefore, our cell strain provides an exceptional model for studying and producing cell-cultured fat.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Evaluation of Fat Accumulation and Adipokine Production during the Long-Term Adipogenic Differentiation of Porcine Intramuscular Preadipocytes and Study of the Influence of Immunobiotics.Cells. 2020 Jul 17;9(7):1715. doi: 10.3390/cells9071715. Cells. 2020. PMID: 32708964 Free PMC article.
-
Technical note: Bovine adipocyte and preadipocyte co-culture as an efficient adipogenic model.J Dairy Sci. 2019 Apr;102(4):3622-3629. doi: 10.3168/jds.2018-15626. Epub 2019 Feb 14. J Dairy Sci. 2019. PMID: 30772027
-
Perspectives on scaling production of adipose tissue for food applications.Biomaterials. 2022 Jan;280:121273. doi: 10.1016/j.biomaterials.2021.121273. Epub 2021 Nov 29. Biomaterials. 2022. PMID: 34933254 Free PMC article.
-
The influence of thiazolidinediones on adipogenesis in vitro and in vivo: potential modifiers of intramuscular adipose tissue deposition in meat animals.J Anim Sci. 2008 Apr;86(14 Suppl):E236-43. doi: 10.2527/jas.2007-0219. Epub 2007 Aug 8. J Anim Sci. 2008. PMID: 17686902 Review.
-
Alternative fat: redefining adipocytes for biomanufacturing cultivated meat.Trends Biotechnol. 2023 May;41(5):686-700. doi: 10.1016/j.tibtech.2022.08.005. Epub 2022 Sep 15. Trends Biotechnol. 2023. PMID: 36117023 Review.
Cited by
-
Cold-Induced DHRS4 Promotes Thermogenesis via Enhanced Fatty Acid β-Oxidation in Porcine Subcutaneous Adipocytes.Animals (Basel). 2025 Apr 22;15(9):1190. doi: 10.3390/ani15091190. Animals (Basel). 2025. PMID: 40362005 Free PMC article.
-
A unique spontaneously immortalised cell line from pig with enhanced adipogenic capacity.NPJ Sci Food. 2025 Apr 20;9(1):52. doi: 10.1038/s41538-025-00413-y. NPJ Sci Food. 2025. PMID: 40254637 Free PMC article.
-
Establishment and characterization of hTERT-immortalized porcine muscle stem cells, and their prospective uses.Food Sci Biotechnol. 2024 Dec 11;34(7):1597-1604. doi: 10.1007/s10068-024-01785-9. eCollection 2025 Apr. Food Sci Biotechnol. 2024. PMID: 40129713
-
Fin Cells as a Promising Seed Cell Source for Sustainable Fish Meat Cultivation.Foods. 2025 Jun 12;14(12):2075. doi: 10.3390/foods14122075. Foods. 2025. PMID: 40565684 Free PMC article.
References
-
- Fernandez X, Mourot J, Lebret B, Gilbert S, Monin G. Influence of intramuscular fat content on lipid composition, sensory qualities and consumer acceptability of cured cooked ham. J. Sci. Food Agric. 2000;80:705–710. doi: 10.1002/(SICI)1097-0010(20000501)80:6<705::AID-JSFA592>3.0.CO;2-8. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

