A prospective open label 2-8 year extension of the randomised controlled ICON trial on the long-term efficacy and safety of occipital nerve stimulation in medically intractable chronic cluster headache
- PMID: 38007947
- PMCID: PMC10755111
- DOI: 10.1016/j.ebiom.2023.104895
A prospective open label 2-8 year extension of the randomised controlled ICON trial on the long-term efficacy and safety of occipital nerve stimulation in medically intractable chronic cluster headache
Abstract
Background: We demonstrated in the randomised controlled ICON study that 48-week treatment of medically intractable chronic cluster headache (MICCH) with occipital nerve stimulation (ONS) is safe and effective. In L-ICON we prospectively evaluate its long-term effectiveness and safety.
Methods: ICON participants were enrolled in L-ICON immediately after completing ICON. Therefore, earlier ICON participants could be followed longer than later ones. L-ICON inclusion was stopped after the last ICON participant was enrolled in L-ICON and followed for ≥2 years by completing six-monthly questionnaires on attack frequency, side effects, subjective improvement and whether they would recommend ONS to others. Primary outcome was the change in mean weekly attack frequency 2 years after completion of the ICON study compared to baseline. Missing values for log-transformed attack-frequency were imputed for up to 5 years of follow-up. Descriptive analyses are presented as (pooled) geometric or arithmetic means and 95% confidence intervals.
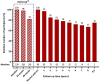
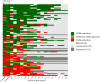
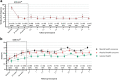
Findings: Of 103 eligible participants, 88 (85%) gave informed consent and 73 (83%) were followed for ≥2 year, 61 (69%) ≥ 3 year, 33 (38%) ≥ 5 years and 3 (3%) ≥ 8.5 years. Mean (±SD) follow-up was 4.2 ± 2.2 years for a total of 370 person years (84% of potentially 442 years). The pooled geometric mean (95% CI) weekly attack frequency remained considerably lower after one (4.2; 2.8-6.3), two (5.1; 3.5-7.6) and five years (4.1; 3.0-5.5) compared to baseline (16.2; 14.4-18.3). Of the 49/88 (56%) ICON ≥50% responders, 35/49 (71%) retained this response and 15/39 (38%) ICON non-responders still became a ≥50% responder for at least half the follow-up period. Most participants (69/88; 78% [0.68-0.86]) reported a subjective improvement from baseline at last follow-up and 70/88 (81% [0.70-0.87]) would recommend ONS to others. Hardware-related surgery was required in 44/88 (50%) participants in 112/122 (92%) events (0.35 person-year-1 [0.28-0.41]). We didn't find predictive factors for effectiveness.
Interpretation: ONS is a safe, well-tolerated and long-term effective treatment for MICCH.
Funding: The Netherlands Organisation for Scientific Research, the Dutch Ministry of Health, the NutsOhra Foundation from the Dutch Health Insurance Companies, and Medtronic.
Keywords: Cluster headache; Medically intractable chronic cluster headache; Neuromodulation; Occipital nerve stimulation.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests WM reports honoraria from Novartis, Teva, AbbVie, Lundbeck and Lilly, and consultancy and lecture fees from Lilly; RF reports consultancy and lecture fees from Novartis, Lundbeck, AbbVie, Lilly and TEVA, and independent support from the Dutch Brain Foundation, Leiden University Fund and Innovation Fund Dutch Healthcare Providers; FH reports consultancy and lecture fees form ABBOTT, Saluda, Grunenthal and Pfizer; RB, EZ, LW, JH, IC and MF report no relevant conflict of interest. No funding was received for the ICON study group.
Figures
References
-
- Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. - PubMed
-
- Hoffmann J., May A. Diagnosis, pathophysiology, and management of cluster headache. Lancet Neurol. 2018;17(1):75–83. - PubMed
-
- Goadsby P.J., Schoenen J., Ferrari M.D., Silberstein S.D., Dodick D. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. 2006;26(9):1168–1170. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

