Renin and renin blockade have no role in complement activity
- PMID: 38008161
- PMCID: PMC10872535
- DOI: 10.1016/j.kint.2023.11.005
Renin and renin blockade have no role in complement activity
Abstract
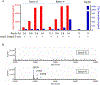
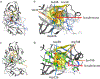
Renin, an aspartate protease, regulates the renin-angiotensin system by cleaving its only known substrate angiotensinogen to angiotensin. Recent studies have suggested that renin may also cleave complement component C3 to activate complement or contribute to its dysregulation. Typically, C3 is cleaved by C3 convertase, a serine protease that uses the hydroxyl group of a serine residue as a nucleophile. Here, we provide seven lines of evidence to show that renin does not cleave C3. First, there is no association between renin plasma levels and C3 levels in patients with C3 Glomerulopathies (C3G) and atypical Hemolytic Uremic Syndrome (aHUS), implying that serum C3 consumption is not increased in the presence of high renin. Second, in vitro tests of C3 conversion to C3b do not detect differences when sera from patients with high renin levels are compared to sera from patients with normal/low renin levels. Third, aliskiren, a renin inhibitor, does not block abnormal complement activity introduced by nephritic factors in the fluid phase. Fourth, aliskiren does not block dysregulated complement activity on cell surfaces. Fifth, recombinant renin from different sources does not cleave C3 even after 24 hours of incubation at 37 °C. Sixth, direct spiking of recombinant renin into sera samples of patients with C3G and aHUS does not enhance complement activity in either the fluid phase or on cell surfaces. And seventh, molecular modeling and docking place C3 in the active site of renin in a position that is not consistent with a productive ground state complex for catalytic hydrolysis. Thus, our study does not support a role for renin in the activation of complement.
Keywords: C3 cleavage; aliskiren; alternative pathway; complement activation; renin.
Copyright © 2023 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure Statement
All authors declare no competing interests.
Figures




References
-
- Peach MJ. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev 1977; 57: 313–370. - PubMed
-
- Griendling KK, Murphy TJ, Alexander RW. Molecular biology of the renin-angiotensin system. Circulation 1993; 87: 1816–1828. - PubMed
-
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311: 507–520. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

