Predicting Visual Acuity Responses to Anti-VEGF Treatment in the Comparison of Age-related Macular Degeneration Treatments Trials Using Machine Learning
- PMID: 38008218
- PMCID: PMC11070304
- DOI: 10.1016/j.oret.2023.11.010
Predicting Visual Acuity Responses to Anti-VEGF Treatment in the Comparison of Age-related Macular Degeneration Treatments Trials Using Machine Learning
Abstract
Purpose: To evaluate multiple machine learning (ML) models for predicting 2-year visual acuity (VA) responses to anti-vascular endothelial growth factor (anti-VEGF) treatment in the Comparison of Age-related Macular Degeneration (AMD) Treatments Trials (CATT) for patients with neovascular AMD (nAMD).
Design: Secondary analysis of public data from a randomized clinical trial.
Participants: A total of 1029 CATT participants who completed 2 years of follow-up with untreated active nAMD and baseline VA between 20/25 and 20/320 in the study eye.
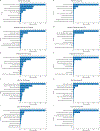
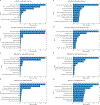
Methods: Five ML models (support vector machine, random forest, extreme gradient boosting, multilayer perceptron neural network, and lasso) were applied to clinical and image data from baseline and weeks 4, 8, and 12 for predicting 4 VA outcomes (≥ 15-letter VA gain, ≥ 15-letter VA loss, VA change from baseline, and actual VA) at 2 years. The CATT data from 1029 participants were randomly split for training (n = 717), from which the models were trained using 10-fold cross-validation, and for final validation on a test data set (n = 312).
Main outcome measures: Performances of ML models were assessed by R2 and mean absolute error (MAE) for predicting VA change from baseline and actual VA at 2 years, by the area under the receiver operating characteristic curve (AUC) for predicting ≥ 15-letter VA gain and loss from baseline.
Results: Using training data up to week 12, the ML models from cross-validation achieved mean R2 of 0.24 to 0.29 (MAE = 9.1-9.8 letters) for predicting VA change and 0.37 to 0.41 (MAE = 9.3-10.2 letters) for predicting actual VA at 2 years. The mean AUCs for predicting ≥ 15-letter VA gain and loss at 2 years was 0.84 to 0.85 and 0.58 to 0.73, respectively. In final validation on the test data set up to week 12, the models had an R2 of 0.33 to 0.38 (MAE = 8.9-9.9 letters) for predicting VA change, an R2 of 0.37 to 0.45 (MAE = 8.8-10.2 letters) for predicting actual VA at 2 years, and AUCs of 0.85 to 0.87 and 0.67 to 0.79 for predicting ≥ 15-letter VA gain and loss, respectively.
Conclusions: Machine learning models have the potential to predict 2-year VA response to anti-VEGF treatment using clinical and imaging features from the loading dose phase, which can aid in decision-making around treatment protocols for patients with nAMD.
Financial disclosure(s): The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Keywords: Age-related macular degeneration; Anti-VEGF therapy; Machine learning; Prediction; Visual acuity.
Copyright © 2023 American Academy of Ophthalmology. All rights reserved.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

