Drug repurposing screens identify Tubercidin as a potent antiviral agent against porcine nidovirus infections
- PMID: 38008220
- PMCID: PMC10730850
- DOI: 10.1016/j.virusres.2023.199275
Drug repurposing screens identify Tubercidin as a potent antiviral agent against porcine nidovirus infections
Abstract
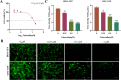
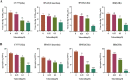
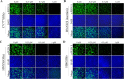
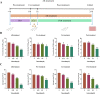
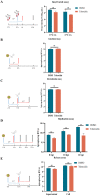
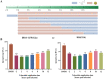
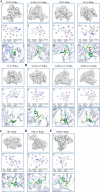
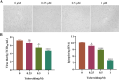
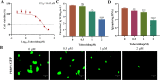
The emergence of new coronaviruses poses a significant threat to animal husbandry and human health. Porcine epidemic diarrhea virus (PEDV) is considered a re-emerging porcine enteric coronavirus, which causes fatal watery diarrhea in piglets. Currently, there are no effective drugs to combat PEDV. Drug repurposing screens have emerged as an attractive strategy to accelerate antiviral drug discovery and development. Here, we screened 206 natural products for antiviral activity using live PEDV infection in Vero cells and identified ten candidate antiviral agents. Among them, Tubercidin, a nucleoside analog derived from Streptomyces tubercidicus, showed promising antiviral activity against PEDV infection. Furthermore, we demonstrated that Tubercidin exhibited significant antiviral activity against both classical and variant PEDV. Time of addition assay showed that Tubercidin displayed a significant inhibitory effect on viral post-entry events but not during other periods. Molecular docking analysis indicated that Tubercidin had better docking efficiency and formed hydrophobic interactions with the active pocket of RNA-dependent RNA polymerase (RdRp) of PEDV and other nidoviruses. Additionally, Tubercidin can effectively suppress other porcine nidoviruses, such as SADS-CoV and PRRSV, demonstrating its broad-spectrum antiviral properties. In summary, our findings provide valuable evidence for the antiviral activity of Tubercidin and offer insights into the development of new strategies for the prevention and treatment of coronavirus infections.
Keywords: Antiviral; Coronavirus; Porcine epidemic diarrhea virus; RNA-dependent RNA polymerase; Tubercidin.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Bergant V., Yamada S., Grass V., Tsukamoto Y., Lavacca T., Krey K., Muhlhofer M.T., Wittmann S., Ensser A., Herrmann A., Vom Hemdt A., Tomita Y., Matsuyama S., Hirokawa T., Huang Y., Piras A., Jakwerth C.A., Oelsner M., Thieme S., Graf A., Krebs S., Blum H., Kummerer B.M., Stukalov A., Schmidt-Weber C.B., Igarashi M., Gramberg T., Pichlmair A., Kato H. Attenuation of SARS-CoV-2 replication and associated inflammation by concomitant targeting of viral and host cap 2′-O-ribose methyltransferases. EMBO J. 2022;41(17) - PMC - PubMed
-
- Cao L., Li Y., Yang S., Li G., Zhou Q., Sun J., Xu T., Yang Y., Liao R., Shi Y., Yang Y., Zhu T., Huang S., Ji Y., Cong F., Luo Y., Zhu Y., Luan H., Zhang H., Chen J., Liu X., Luo R., Liu L., Wang P., Yu Y., Xing F., Ke B., Zheng H., Deng X., Zhang W., Lin C., Shi M., Li C.M., Zhang Y., Zhang L., Dai J., Lu H., Zhao J., Zhang X., Guo D. The adenosine analog prodrug ATV006 is orally bioavailable and has preclinical efficacy against parental SARS-CoV-2 and variants. Sci. Transl. Med. 2022;14(661):eabm7621. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

