Hsp90 β is critical for the infection of severe fever with thrombocytopenia syndrome virus
- PMID: 38008382
- PMCID: PMC10877427
- DOI: 10.1016/j.virs.2023.11.008
Hsp90 β is critical for the infection of severe fever with thrombocytopenia syndrome virus
Abstract
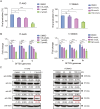
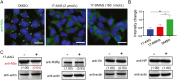
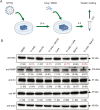
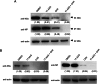
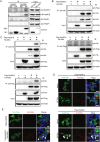
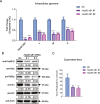
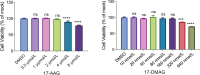
Severe fever with thrombocytopenia syndrome (SFTS) caused by the SFTS virus (SFTSV) is an emerging disease in East Asia with a fatality rate of up to 30%. However, the viral-host interaction of SFTSV remains largely unknown. The heat-shock protein 90 (Hsp90) family consists of highly conserved chaperones that fold and remodel proteins and has a broad impact on the infection of many viruses. Here, we showed that Hsp90 is an important host factor involved in SFTSV infection. Hsp90 inhibitors significantly reduced SFTSV replication, viral protein expression, and the formation of inclusion bodies consisting of nonstructural proteins (NSs). Among viral proteins, NSs appeared to be the most reduced when Hsp90 inhibitors were used, and further analysis showed that their translation was affected. Co-immunoprecipitation of NSs with four isomers of Hsp90 showed that Hsp90 β specifically interacted with them. Knockdown of Hsp90 β expression also inhibited replication of SFTSV. These results suggest that Hsp90 β plays a critical role during SFTSV infection and could be a potential target for the development of drugs against SFTS.
Keywords: Heat-shock protein 90; Host-virus interaction; Hsp90 β; Nonstructural protein; Severe fever with thrombocytopenia syndrome virus (SFTSV).
Copyright © 2023 The Authors. Publishing services by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflict of interest. Prof. Fei Deng is an editorial board member for Virologica Sinica and was not involved in the editorial review or the decision to publish this article.
Figures

Similar articles
-
Two Conserved Amino Acids within the NSs of Severe Fever with Thrombocytopenia Syndrome Phlebovirus Are Essential for Anti-interferon Activity.J Virol. 2018 Sep 12;92(19):e00706-18. doi: 10.1128/JVI.00706-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021900 Free PMC article.
-
Nonstructural Protein NSs Activates Inflammasome and Pyroptosis through Interaction with NLRP3 in Human Microglial Cells Infected with Severe Fever with Thrombocytopenia Syndrome Bandavirus.J Virol. 2022 Jul 13;96(13):e0016722. doi: 10.1128/jvi.00167-22. Epub 2022 Jun 13. J Virol. 2022. PMID: 35695505 Free PMC article.
-
Species-Specific Pathogenicity of Severe Fever with Thrombocytopenia Syndrome Virus Is Determined by Anti-STAT2 Activity of NSs.J Virol. 2019 May 1;93(10):e02226-18. doi: 10.1128/JVI.02226-18. Print 2019 May 15. J Virol. 2019. PMID: 30814285 Free PMC article.
-
Severe fever with thrombocytopenia syndrome and its pathogen SFTSV.Microbes Infect. 2015 Feb;17(2):149-54. doi: 10.1016/j.micinf.2014.12.002. Epub 2014 Dec 11. Microbes Infect. 2015. PMID: 25498868 Review.
-
Overview of the immunological mechanism underlying severe fever with thrombocytopenia syndrome (Review).Int J Mol Med. 2022 Sep;50(3):118. doi: 10.3892/ijmm.2022.5174. Epub 2022 Jul 20. Int J Mol Med. 2022. PMID: 35856413 Free PMC article. Review.
References
-
- Abudurexiti A., Adkins S., Alioto D., Alkhovsky S.V., Avšič-Županc T., Ballinger M.J., Bente D.A., Beer M., Bergeron É., Blair C.D., Briese T., Buchmeier M.J., Burt F.J., Calisher C.H., Cháng C., Charrel R.N., Choi I.R., Clegg J.C.S., De La Torre J.C., De Lamballerie X., Dèng F., Di Serio F., Digiaro M., Drebot M.A., Duàn X., Ebihara H., Elbeaino T., Ergünay K., Fulhorst C.F., Garrison A.R., Gāo G.F., Gonzalez J.J., Groschup M.H., Günther S., Haenni A.L., Hall R.A., Hepojoki J., Hewson R., Hú Z., Hughes H.R., Jonson M.G., Junglen S., Klempa B., Klingström J., Kòu C., Laenen L., Lambert A.J., Langevin S.A., Liu D., Lukashevich I.S., Luò T., Lǚ C., Maes P., De Souza W.M., Marklewitz M., Martelli G.P., Matsuno K., Mielke-Ehret N., Minutolo M., Mirazimi A., Moming A., Mühlbach H.P., Naidu R., Navarro B., Nunes M.R.T., Palacios G., Papa A., Pauvolid-Corrêa A., Pawęska J.T., Qiáo J., Radoshitzky S.R., Resende R.O., Romanowski V., Sall A.A., Salvato M.S., Sasaya T., Shěn S., Shí X., Shirako Y., Simmonds P., Sironi M., Song J.W., Spengler J.R., Stenglein M.D., Sū Z., Sūn S., Táng S., Turina M., Wáng B., Wáng C., Wáng H., Wáng J., Wèi T., Whitfield A.E., Zerbini F.M., Zhāng J., Zhāng L., Zhāng Y., Zhang Y.Z., Zhāng Y., Zhou X., Zhū L., Kuhn J.H. Taxonomy of the order Bunyavirales: update 2019. Arch. Virol. 2019;164:1949–1965. - PMC - PubMed
-
- Basta S., Stoessel R., Basler M., Van Den Broek M., Groettrup M. Cross-presentation of the long-lived lymphocytic choriomeningitis virus nucleoprotein does not require neosynthesis and is enhanced via heat shock proteins. J. Immunol. 2005;175:796–805. - PubMed
-
- Chen B., Piel W.H., Gui L., Bruford E., Monteiro A. The HSP90 family of genes in the human genome: insights into their divergence and evolution. Genomics. 2005;86:627–637. - PubMed
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources

