A landscape on disorders following different COVID-19 vaccination: a systematic review of Iranian case reports
- PMID: 38008729
- PMCID: PMC10676592
- DOI: 10.1186/s40001-023-01531-7
A landscape on disorders following different COVID-19 vaccination: a systematic review of Iranian case reports
Abstract
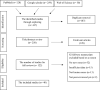
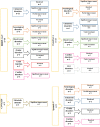
There have been massive studies to develop an effective vaccine against SARS-CoV-2 which fortunately led to manage the recent pandemic, COVID-19. According to the quite rapidly developed vaccines in a fast window time, large investigations to assess the probable vaccine-related adverse events are crucially required. COVID-19 vaccines are available of different platforms and the primary clinical trials results presented acceptable safety profile of the approved vaccines. Nevertheless, the long-term assessment of the adverse events or rare conditions need to be investigated. The present systematic review, aimed at classification of probable vaccine-related unsolicited adverse events in Iranian population through the data collection of the published case report studies.The related published case reports were explored via PubMed, Web of Science and Google scholar according to the available published data up to 14th Dec, 2022 using PRISMA guideline. Out of 437 explored studies, the relevant data were fully investigated which totally led to 40 studies, including 64 case reports with a new onset of a problem post-vaccination. The cases were then classified according to the various items, such as the type of adverse event and COVID-19 vaccines.The reported COVID-19 vaccines in the studied cases included BBIBP-CorV, ChAdOx1-S, Sputnik V and COVAXIN. The results showed that the adverse events presented in 8 different categories, including cutaneous involvements in 43.7% (n = 28), neurologic problems (n = 16), blood/vessel involvement (n = 6), cardiovascular involvement (n = 5), ocular disorders (n = 4), liver disorder/failure (n = 2), graft rejection (n = 2) and one metabolic disorder. Notably, almost 60% of the cases had no comorbidities. Moreover, the obtained data revealed nearly half of the incidences occurred after the first dose of injection and the median duration of improvement after the symptom was 10 days (range: 2-120). In addition, 73% of all the cases were either significantly improved or fully recovered. Liver failure following ChAdOx1-S vaccination was the most serious vaccine adverse event which led to death in two individuals with no related medical history.Although the advantages of COVID-19 vaccination is undoubtedly significant, individuals including with a history of serious disease, comorbidities and immunodeficiency conditions should be vaccinated with the utmost caution. This study provides a comprehensive overview and clinical implications of possible vaccine-related adverse events which should be considered in further vaccination strategies. Nevertheless, there might be a bias regarding potential under-reporting and missing data of the case reports included in the present study. Although the reported data are not proven to be the direct vaccination outcomes and could be a possible immune response over stimulation, the people the population with a medium/high risk should be monitored after getting vaccinated against COVID-19 of any platforms. This could be achieved by a carefull attention to the subjects ' medical history and also through consulting with healthcare providers before vaccination.
Keywords: Adverse event; Organ involvement; SARS-CoV-2; Vaccine monitoring.
© 2023. The Author(s).
Conflict of interest statement
There are no competing interests to be declared.
Figures
References
-
- Mostafa Salehi-Vaziri TJ, Farahmand B, Fotouhi F, Banifazl M, Pouriayevali MH, Larijani MS, Afzali N, Ramezani A. Clinical Characteristics of SARS-CoV-2 by Re-infection Vs. reactivation: a case series from Iran. Eur J Clin Microbiol Infect Dis. 2021 doi: 10.1007/s10096-021-04221-6. - DOI - PMC - PubMed
-
- Larijani MS, Ramezani A, Shirazi MMA, Bolhassani A, Pouriayevali MH, Shahbazi S, et al. Evaluation of transduced dendritic cells expressing HIV-1 p24-Nef antigens in HIV-specific cytotoxic T cells induction as a therapeutic candidate vaccine. Virus Res. 2021 doi: 10.1016/j.virusres.2021.198403. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous