Exploiting innate immunity for cancer immunotherapy
- PMID: 38008741
- PMCID: PMC10680233
- DOI: 10.1186/s12943-023-01885-w
Exploiting innate immunity for cancer immunotherapy
Abstract
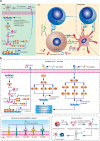
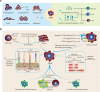
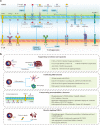
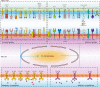
Immunotherapies have revolutionized the treatment paradigms of various types of cancers. However, most of these immunomodulatory strategies focus on harnessing adaptive immunity, mainly by inhibiting immunosuppressive signaling with immune checkpoint blockade, or enhancing immunostimulatory signaling with bispecific T cell engager and chimeric antigen receptor (CAR)-T cell. Although these agents have already achieved great success, only a tiny percentage of patients could benefit from immunotherapies. Actually, immunotherapy efficacy is determined by multiple components in the tumor microenvironment beyond adaptive immunity. Cells from the innate arm of the immune system, such as macrophages, dendritic cells, myeloid-derived suppressor cells, neutrophils, natural killer cells, and unconventional T cells, also participate in cancer immune evasion and surveillance. Considering that the innate arm is the cornerstone of the antitumor immune response, utilizing innate immunity provides potential therapeutic options for cancer control. Up to now, strategies exploiting innate immunity, such as agonists of stimulator of interferon genes, CAR-macrophage or -natural killer cell therapies, metabolic regulators, and novel immune checkpoint blockade, have exhibited potent antitumor activities in preclinical and clinical studies. Here, we summarize the latest insights into the potential roles of innate cells in antitumor immunity and discuss the advances in innate arm-targeted therapeutic strategies.
Keywords: Cancer immunotherapy; Chimeric antigen receptor; Dendritic cell; Innate immunity; Macrophage; Myeloid-derived suppressor cell; Natural killer cell; Neutrophil.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

