A rat study on the PTEN expression in ovarian tissue in PCOS and folliculogenesis
- PMID: 38008769
- PMCID: PMC10679187
- DOI: 10.1038/s41598-023-47809-y
A rat study on the PTEN expression in ovarian tissue in PCOS and folliculogenesis
Abstract
The objective of this investigation was to examine alterations in PTEN expression within ovarian tissue in a rat model of polycystic ovary syndrome (PCOS). The analysis also encompassed the examination of PTEN alterations in the ovarian tissue throughout the process of folliculogenesis in rats with normal ovulatory cycles. The study involved 12 adult female Sprague‒Dawley rats randomly assigned to the letrozole-induced polycystic ovary syndrome (PCOS) group as part of an animal-based research endeavour. The sections derived from the ovaries were subjected to immunohistochemical staining for PTEN. The evaluation of PTEN staining levels in ovarian tissues was conducted using electron microscopy. Follicle counts, as well as hormonal and biochemical analyses (serum luteinising hormone (LH), follicle-stimulating hormone (FSH), anti-Müllerian hormone (AMH), testosterone, oestradiol levels and serum glucose, triglyceride, HDL and LDL-cholesterol levels), were conducted to provide evidence of the manifestation of polycystic ovary syndrome (PCOS) in rats. The number of primordial and Graafian follicles in the PCOS group decreased significantly, and the number of primary, secondary and antral follicles increased significantly. PTEN expression was found to be significantly higher in the PCOS group than in the control group in the primordial follicle oocyte cytoplasm, primordial follicle granulosa cells, primary follicle oocyte cytoplasm, primary follicle granulosa cells, antral follicle oocyte cytoplasm, antral follicle granulosa cells, and corpus luteum (p = 0.007, p = 0.001, p = 0.001, p = 0.001, p = 0.001, p = 0.002, and p = 0.018, respectively). In the non-PCOS group, a time-dependent comparison of the amount of oocyte cytoplasm and PTEN staining in granulosa cells of the oocytes at different stages of development was performed. While the follicles were developing from the primordial follicle to the primary and antral follicle, the amount of PTEN staining in the oocyte cytoplasm decreased, whereas the PTEN activity in the granulosa cells increased as the oocyte developed (p = 0.001 and p = 0.001, respectively). The current investigation demonstrated changes in PTEN expression in ovarian tissue throughout the course of normal folliculogenesis, as well as in instances of disrupted folliculogenesis, with a focus on rats with PCOS.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



 ), Multilaminar Primary Follicle (MPF), Antral Follicle (AF), Oocyte Cytoplasm (
), Multilaminar Primary Follicle (MPF), Antral Follicle (AF), Oocyte Cytoplasm ( ), Corpus Luteum (CL), Stroma (
), Corpus Luteum (CL), Stroma ( ) (DAP—Hematoxylin × 100). (IIIa) Primordialfolicula (
) (DAP—Hematoxylin × 100). (IIIa) Primordialfolicula ( ), Multilaminar Primer Follicle (MPF), Oocyte Cytoplasm (
), Multilaminar Primer Follicle (MPF), Oocyte Cytoplasm ( ) (DAP—Hematoxylin × 400). (IIIb) Primordial Follicle- Primary Follicle Transition (
) (DAP—Hematoxylin × 400). (IIIb) Primordial Follicle- Primary Follicle Transition ( ) Unilaminar Primary Follicle (UPF) (DAP—Hematoxylin × 400). PTEN immunoreactivity was strong in the oocyte cytoplasm of the primordial follicle, weak in the granulosa cells surrounding the oocyte, and varying from weak to moderate in the oocyte cytoplasm of the cubic granulosa cells. PTEN involvement was moderate in the surrounding unilaminar primary follicle granulosa cells and moderate to strong in the multilaminar primary and antral follicle granulosa cells. In the corpus luteum, PTEN immunoreactivity was moderate.
) Unilaminar Primary Follicle (UPF) (DAP—Hematoxylin × 400). PTEN immunoreactivity was strong in the oocyte cytoplasm of the primordial follicle, weak in the granulosa cells surrounding the oocyte, and varying from weak to moderate in the oocyte cytoplasm of the cubic granulosa cells. PTEN involvement was moderate in the surrounding unilaminar primary follicle granulosa cells and moderate to strong in the multilaminar primary and antral follicle granulosa cells. In the corpus luteum, PTEN immunoreactivity was moderate.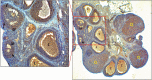

Similar articles
-
The regulation and signalling of anti-Müllerian hormone in human granulosa cells: relevance to polycystic ovary syndrome.Hum Reprod. 2019 Dec 1;34(12):2467-2479. doi: 10.1093/humrep/dez214. Hum Reprod. 2019. PMID: 31735954
-
Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary.Hum Reprod Update. 2016 Nov;22(6):709-724. doi: 10.1093/humupd/dmw027. Epub 2016 Aug 27. Hum Reprod Update. 2016. PMID: 27566840 Review.
-
Activation of TGF-β1/Smad3 signaling pathway inhibits the development of ovarian follicle in polycystic ovary syndrome by promoting apoptosis of granulosa cells.J Cell Physiol. 2019 Jul;234(7):11976-11985. doi: 10.1002/jcp.27854. Epub 2018 Dec 7. J Cell Physiol. 2019. PMID: 30536903
-
Early postnatal methoxychlor exposure inhibits folliculogenesis and stimulates anti-Mullerian hormone production in the rat ovary.J Endocrinol. 2006 Dec;191(3):549-58. doi: 10.1677/joe.1.06592. J Endocrinol. 2006. PMID: 17170213
-
Gonadotropin Activity during Early Folliculogenesis and Implications for Polycystic Ovarian Syndrome and Premature Ovarian Insufficiency: A Narrative Review.Int J Mol Sci. 2024 Jul 9;25(14):7520. doi: 10.3390/ijms25147520. Int J Mol Sci. 2024. PMID: 39062762 Free PMC article. Review.
Cited by
-
Integrated Transcriptomics and Single-Cell RNA Sequencing Analyses Reveal the Potential Role of Obesity-Related Genes in Polycystic Ovary Syndrome.Reprod Sci. 2025 Aug 29. doi: 10.1007/s43032-025-01968-7. Online ahead of print. Reprod Sci. 2025. PMID: 40880058
-
The effect of aqueous Stevia rebaudiana extract and clomiphene citrate on the expression of GDF9, PTEN, BMP15 genes, and antioxidant levels in a letrozole-induced polycystic ovary syndrome model in Wistar rats.J Ovarian Res. 2025 Jul 4;18(1):144. doi: 10.1186/s13048-025-01719-x. J Ovarian Res. 2025. PMID: 40615883 Free PMC article.
-
Reduced miR-338-3p contributes to polycystic ovarian syndrome by inhibiting proliferation and enhancing apoptosis.Hereditas. 2025 Jul 12;162(1):126. doi: 10.1186/s41065-025-00498-1. Hereditas. 2025. PMID: 40652291 Free PMC article.
-
Evaluating the impact of coenzyme Q10 and high-intensity interval training on lactate threshold and Plasma blood gases in rats: a randomized controlled trial.Eur J Appl Physiol. 2025 Aug;125(8):2185-2196. doi: 10.1007/s00421-025-05756-8. Epub 2025 Mar 18. Eur J Appl Physiol. 2025. PMID: 40100404 Free PMC article.
-
SNHG12 downregulation induces follicular dysplasia by modulating the glycolysis of granulosa cell in polycystic ovary syndrome.Front Cell Dev Biol. 2025 May 23;13:1585987. doi: 10.3389/fcell.2025.1585987. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40486908 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

