MSCs overexpressing GDNF restores brain structure and neurological function in rats with intracerebral hemorrhage
- PMID: 38008847
- PMCID: PMC10678901
- DOI: 10.1186/s43556-023-00159-7
MSCs overexpressing GDNF restores brain structure and neurological function in rats with intracerebral hemorrhage
Abstract
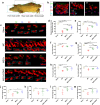
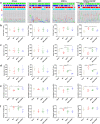
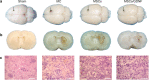
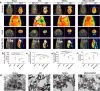
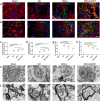
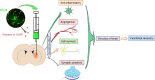
Mesenchymal stem cells (MSCs) have been applied in transplantation to treat intracerebral hemorrhage (ICH) but with limited efficacy. Accumulated evidence has shown that glial cell-derived neurotrophic factor (GDNF) plays a crucial part in neuronal protection and functional recovery of the brain after ICH; however, GDNF has difficulty crossing the blood-brain barrier, which limits its application. In this study, we investigated the influences of MSCs overexpressing GDNF (MSCs/GDNF) on the brain structure as well as gait of rats after ICH and explored the possible mechanisms. We found that cell transplantation could reverse the neurological dysfunction and brain damage caused by ICH to a certain extent, and MSCs/GDNF transplantation was superior to MSCs transplantation. Moreover, Transplantation of MSCs overexpressing GDNF effectively reduced the volume of bleeding foci and increased the level of glucose uptake in rats with ICH, which could be related to improving mitochondrial quality. Furthermore, GDNF produced by transplanted MSCs/GDNF further inhibited neuroinflammation, improved mitochondrial quality and function, promoted angiogenesis and the survival of neurons and oligodendrocytes, and enhanced synaptic plasticity in ICH rats when compared with simple MSC transplantation. Overall, our data indicate that GDNF overexpression heightens the curative effect of MSC implantation in treating rats following ICH.
Keywords: Glial cell-derived neurotrophic factor; Intracerebral hemorrhage; Mesenchymal stem cells; Neurological recovery; Synaptic plasticity.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Effects of GDNF-Transfected Marrow Stromal Cells on Rats with Intracerebral Hemorrhage.J Stroke Cerebrovasc Dis. 2019 Sep;28(9):2555-2562. doi: 10.1016/j.jstrokecerebrovasdis.2019.06.002. Epub 2019 Jun 24. J Stroke Cerebrovasc Dis. 2019. PMID: 31248739
-
Human neural stem cells overexpressing glial cell line-derived neurotrophic factor in experimental cerebral hemorrhage.Gene Ther. 2009 Sep;16(9):1066-76. doi: 10.1038/gt.2009.51. Epub 2009 Jun 25. Gene Ther. 2009. PMID: 19554035
-
Priming Wharton's jelly-derived mesenchymal stromal/stem cells with ROCK inhibitor improves recovery in an intracerebral hemorrhage model.J Cell Biochem. 2015 Feb;116(2):310-9. doi: 10.1002/jcb.24969. J Cell Biochem. 2015. PMID: 25185536
-
Mesenchymal Stem Cell Application and Its Therapeutic Mechanisms in Intracerebral Hemorrhage.Front Cell Neurosci. 2022 Jun 13;16:898497. doi: 10.3389/fncel.2022.898497. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35769327 Free PMC article. Review.
-
Mesenchymal Stem Cells Transplantation in Intracerebral Hemorrhage: Application and Challenges.Front Cell Neurosci. 2021 Mar 24;15:653367. doi: 10.3389/fncel.2021.653367. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33841103 Free PMC article. Review.
Cited by
-
Remote ischemic preconditioning plays a neuroprotective role in cerebral ischemia-reperfusion mice by inhibiting mitophagy.Heliyon. 2024 Oct 10;10(20):e39076. doi: 10.1016/j.heliyon.2024.e39076. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39640619 Free PMC article.
References
-
- Qureshi AI, Quresh AI, Huang W, Lobanova I, Barsan WG, Hanley DF, et al. Outcomes of intensive systolic blood pressure reduction in patients with intracerebral hemorrhage and excessively high initial systolic blood pressure: Post Hoc analysis of a randomized clinical trial. JAMA Neurol. 2020;77(11):1355–65. doi: 10.1001/jamaneurol.2020.3075. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
