Validation of vaginal microbiome proxies for in vitro experiments that biomimic Lactobacillus-dominant vaginal cultures
- PMID: 38009054
- PMCID: PMC10691763
- DOI: 10.1111/aji.13797
Validation of vaginal microbiome proxies for in vitro experiments that biomimic Lactobacillus-dominant vaginal cultures
Abstract
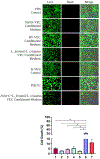
The vaginal microbiome includes diverse microbiota dominated by Lactobacillus [L.] spp. that protect against infections, modulate inflammation, and regulate vaginal homeostasis. Because it is challenging to incorporate vaginal microbiota into in vitro models, including organ-on-a-chip systems, we assessed microbial metabolites as reliable proxies in addition to traditional vaginal epithelial cultures (VECs). Human immortalized VECs cultured on transwells with an air-liquid interface generated stratified cell layers colonized by transplanted healthy microbiomes (L. jensenii- or L. crispatus-dominant) or a community representing bacterial vaginosis (BV). After 48-h, a qPCR array confirmed the expected donor community profiles. Pooled apical and basal supernatants were subjected to metabolomic analysis (untargeted mass spectrometry) followed by ingenuity pathways analysis (IPA). To determine the bacterial metabolites' ability to recreate the vaginal microenvironment in vitro, pooled bacteria-free metabolites were added to traditional VEC cultures. Cell morphology, viability, and cytokine production were assessed. IPA analysis of metabolites from colonized samples contained fatty acids, nucleic acids, and sugar acids that were associated with signaling networks that contribute to secondary metabolism, anti-fungal, and anti-inflammatory functions indicative of a healthy vaginal microbiome compared to sterile VEC transwell metabolites. Pooled metabolites did not affect cell morphology or induce cell death (∼5.5%) of VEC cultures (n = 3) after 72-h. However, metabolites created an anti-inflammatory milieu by increasing IL-10 production (p = .06, T-test) and significantly suppressing pro-inflammatory IL-6 (p = .0001), IL-8 (p = .009), and TNFα (p = .0007) compared to naïve VEC cultures. BV VEC conditioned-medium did not affect cell morphology nor viability; however, it induced a pro-inflammatory environment by elevating levels of IL-6 (p = .023), IL-8 (p = .031), and TNFα (p = .021) when compared to L.-dominate microbiome-conditioned medium. VEC transwells provide a suitable ex vivo system to support the production of bacterial metabolites consistent with the vaginal milieu allowing subsequent in vitro studies with enhanced accuracy and utility.
Keywords: anti-inflammatory; metabolites; vaginal epithelial cells.
© 2023 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

