Cell-Matrix Elastocapillary Interactions Drive Pressure-based Wetting of Cell Aggregates
- PMID: 38009085
- PMCID: PMC10673637
- DOI: 10.1103/physrevx.12.031027
Cell-Matrix Elastocapillary Interactions Drive Pressure-based Wetting of Cell Aggregates
Abstract
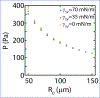
Cell-matrix interfacial energies and the energies of matrix deformations may be comparable on cellular length-scales, yet how capillary effects influence tis sue shape and motion are unknown. In this work, we induce wetting (spreading and migration) of cell aggregates, as models of active droplets onto adhesive substrates of varying elasticity and correlate the dynamics of wetting to the balance of interfacial tensions. Upon wetting rigid substrates, cell-substrate tension drives outward expansion of the monolayer. By contrast, upon wetting compliant substrates, cell substrate tension is attenuated and aggregate capillary forces contribute to internal pressures that drive expansion. Thus, we show by experiments, data-driven modeling and computational simulations that myosin-driven 'active elasto-capillary' effects enable adaptation of wetting mechanisms to substrate rigidity and introduce a novel, pressure-based mechanism for guiding collective cell motion.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
