Mitochondrial influences on smooth muscle phenotype
- PMID: 38009196
- PMCID: PMC11932527
- DOI: 10.1152/ajpcell.00354.2023
Mitochondrial influences on smooth muscle phenotype
Abstract
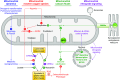
Smooth muscle cells transition reversibly between contractile and noncontractile phenotypes in response to diverse influences, including many from mitochondria. Numerous molecules including myocardin, procontractile miRNAs, and the mitochondrial protein prohibitin-2 promote contractile differentiation; this is opposed by mitochondrial reactive oxygen species (mtROS), high lactate concentrations, and metabolic reprogramming induced by mitophagy and/or mitochondrial fission. A major pathway through which vascular pathologies such as oncogenic transformation, pulmonary hypertension, and atherosclerosis cause loss of vascular contractility is by enhancing mitophagy and mitochondrial fission with secondary effects on smooth muscle phenotype. Proproliferative miRNAs and the mitochondrial translocase TOMM40 also attenuate contractile differentiation. Hypoxia can initiate loss of contractility by enhancing mtROS and lactate production while simultaneously depressing mitochondrial respiration. Mitochondria can reduce cytosolic calcium by moving it across the inner mitochondrial membrane via the mitochondrial calcium uniporter, and then through mitochondria-associated membranes to and from calcium stores in the sarcoplasmic/endoplasmic reticulum. Through these effects on calcium, mitochondria can influence multiple calcium-sensitive nuclear transcription factors and genes, some of which govern smooth muscle phenotype, and possibly also the production of genomically encoded mitochondrial proteins and miRNAs (mitoMirs) that target the mitochondria. In turn, mitochondria also can influence nuclear transcription and mRNA processing through mitochondrial retrograde signaling, which is currently a topic of intensive investigation. Mitochondria also can signal to adjacent cells by contributing to the content of exosomes. Considering these and other mechanisms, it is becoming increasingly clear that mitochondria contribute significantly to the regulation of smooth muscle phenotype and differentiation.
Keywords: metabolic reprogramming; mitoMirs; mitochondria-associated membranes; mitochondrial calcium; mitochondrial retrograde signaling.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
Endoplasmic reticulum Ca2+ release causes Rieske iron-sulfur protein-mediated mitochondrial ROS generation in pulmonary artery smooth muscle cells.Biosci Rep. 2019 Dec 20;39(12):BSR20192414. doi: 10.1042/BSR20192414. Biosci Rep. 2019. Retraction in: Biosci Rep. 2020 Apr 30;40(4):BSR-20192414_RET. doi: 10.1042/BSR-20192414_RET. PMID: 31710081 Free PMC article. Retracted.
-
Mitochondrial regulation of sarcoplasmic reticulum Ca2+ content in vascular smooth muscle cells.Circ Res. 2009 Jan 2;104(1):104-12. doi: 10.1161/CIRCRESAHA.108.180612. Epub 2008 Nov 20. Circ Res. 2009. PMID: 19023135
-
Cross Talk Between Mitochondrial Reactive Oxygen Species and Sarcoplasmic Reticulum Calcium in Pulmonary Arterial Smooth Muscle Cells.Adv Exp Med Biol. 2017;967:289-298. doi: 10.1007/978-3-319-63245-2_17. Adv Exp Med Biol. 2017. PMID: 29047093 Review.
-
Mitochondrial Protein Poldip2 (Polymerase Delta Interacting Protein 2) Controls Vascular Smooth Muscle Differentiated Phenotype by O-Linked GlcNAc (N-Acetylglucosamine) Transferase-Dependent Inhibition of a Ubiquitin Proteasome System.Circ Res. 2020 Jan 3;126(1):41-56. doi: 10.1161/CIRCRESAHA.119.315932. Epub 2019 Oct 28. Circ Res. 2020. PMID: 31656131 Free PMC article.
-
Deciphering the role of MitomiRs in cancer: A comprehensive review.Mitochondrion. 2023 May;70:118-130. doi: 10.1016/j.mito.2023.04.004. Epub 2023 Apr 28. Mitochondrion. 2023. PMID: 37120081 Review.
Cited by
-
Loss-of-function mitochondrial DNA polymerase gamma variants cause vascular smooth muscle cells to secrete a diffusible mitogenic factor.Front Physiol. 2025 Feb 17;15:1488248. doi: 10.3389/fphys.2024.1488248. eCollection 2024. Front Physiol. 2025. PMID: 40034369 Free PMC article.
-
The Ferroptosis-Mitochondrial Axis in Depression: Unraveling the Feedforward Loop of Oxidative Stress, Metabolic Homeostasis Dysregulation, and Neuroinflammation.Antioxidants (Basel). 2025 May 20;14(5):613. doi: 10.3390/antiox14050613. Antioxidants (Basel). 2025. PMID: 40427494 Free PMC article. Review.
-
Stress-Related Roles of Exosomes and Exosomal miRNAs in Common Neuropsychiatric Disorders.Int J Mol Sci. 2024 Jul 29;25(15):8256. doi: 10.3390/ijms25158256. Int J Mol Sci. 2024. PMID: 39125827 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- P01 HD083132/HD/NICHD NIH HHS/United States
- R01HL135786/HHS | NIH | Office of Extramural Research, National Institutes of Health (OER)
- P01HD083132/HHS | NIH | Office of Extramural Research, National Institutes of Health (OER)
- R01 HL149608/HL/NHLBI NIH HHS/United States
- R01 HL135786/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources

