Molecular Diagnostics and [18F]FDG-PET/CT in Indeterminate Thyroid Nodules: Complementing Techniques or Waste of Valuable Resources?
- PMID: 38009209
- PMCID: PMC10818054
- DOI: 10.1089/thy.2023.0337
Molecular Diagnostics and [18F]FDG-PET/CT in Indeterminate Thyroid Nodules: Complementing Techniques or Waste of Valuable Resources?
Abstract
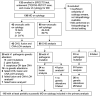
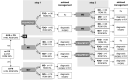
Background: An accurate preoperative workup of cytologically indeterminate thyroid nodules (ITN) may rule out malignancy and avoid diagnostic surgery for benign nodules. This study assessed the performance of molecular diagnostics (MD) and 2-[18F]fluoro-2-deoxy-d-glucose ([18F]FDG)-positron emission tomography/computed tomography (PET/CT) in ITN, including their combined use, and explored whether molecular alterations drive the differences in [18F]FDG uptake among benign nodules. Methods: Adult, euthyroid patients with a Bethesda III or IV thyroid nodule were prospectively included in this multicenter study. They all underwent MD and an [18F]FDG-PET/CT scan of the neck. MD was performed using custom next-generation sequencing panels for somatic mutations, gene fusions, and copy number alterations and loss of heterozygosity. Sensitivity, specificity, negative and positive predictive value (NPV, PPV), and benign call rate (BCR) were assessed for MD and [18F]FDG-PET/CT separately and for a combined approach using both techniques. Results: In 115 of the 132 (87%) included patients, MD yielded a diagnostic result on cytology. Sensitivity, specificity, NPV, PPV, and BCR were 80%, 69%, 91%, 48%, and 57% for MD, and 93%, 41%, 95%, 36%, and 32% for [18F]FDG-PET/CT, respectively. When combined, sensitivity and specificity were 95% and 44% for a double-negative test (i.e., negative MD plus negative [18F]FDG-PET/CT) and 68% and 86% for a double-positive test, respectively. Concordance was 63% (82/130) between MD and [18F]FDG-PET/CT. There were more MD-positive nodules among the [18F]FDG-positive benign nodules (25/59, 42%, including 11 (44%) isolated RAS mutations) than among the [18F]FDG-negative benign nodules (7/30, 19%, p = 0.02). In oncocytic ITN, the BCR of [18F]FDG-PET/CT was mere 3% and MD was the superior technique. Conclusions: MD and [18F]FDG-PET/CT are both accurate rule-out tests when unresected nodules that remain unchanged on ultrasound follow-up are considered benign. It may vary worldwide which test is considered most suitable, depending on local availability of diagnostics, expertise, and cost-effectiveness considerations. Although complementary, the benefits of their combined use may be confined when therapeutic consequences are considered, and should therefore not routinely be recommended. In nononcocytic ITN, sequential testing may be considered in case of a first-step MD negative test to confirm that withholding diagnostic surgery is oncologically safe. In oncocytic ITN, after further validation studies, MD might be considered. Clinical Trial Registration: This trial is registered with ClinicalTrials.gov: NCT02208544 (August 5, 2014), https://clinicaltrials.gov/ct2/show/NCT02208544.
Keywords: [18F]FDG-PET/CT; indeterminate cytology; molecular diagnostics; thyroid carcinoma; thyroid nodules.
Conflict of interest statement
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Figures
Similar articles
-
[18F]FDG-PET/CT to prevent futile surgery in indeterminate thyroid nodules: a blinded, randomised controlled multicentre trial.Eur J Nucl Med Mol Imaging. 2022 May;49(6):1970-1984. doi: 10.1007/s00259-021-05627-2. Epub 2022 Jan 4. Eur J Nucl Med Mol Imaging. 2022. PMID: 34981165 Free PMC article. Clinical Trial.
-
Quantitative classification and radiomics of [18F]FDG-PET/CT in indeterminate thyroid nodules.Eur J Nucl Med Mol Imaging. 2022 Jun;49(7):2174-2188. doi: 10.1007/s00259-022-05712-0. Epub 2022 Feb 9. Eur J Nucl Med Mol Imaging. 2022. PMID: 35138444 Free PMC article. Clinical Trial.
-
[18F]FDG Uptake and Expression of Immunohistochemical Markers Related to Glycolysis, Hypoxia, and Proliferation in Indeterminate Thyroid Nodules.Mol Imaging Biol. 2023 Jun;25(3):483-494. doi: 10.1007/s11307-022-01776-4. Epub 2022 Oct 17. Mol Imaging Biol. 2023. PMID: 36253663 Free PMC article. Clinical Trial.
-
[Preoperative evaluation of cytologically indeterminate thyroid nodules with 18F-FDG PET].Arq Bras Endocrinol Metabol. 2008 Oct;52(7):1176-83. doi: 10.1590/s0004-27302008000700015. Arq Bras Endocrinol Metabol. 2008. PMID: 19082307 Review. Portuguese.
-
The role of 18F-fluorodeoxyglucose positron emission tomography in thyroid neoplasms.Oncologist. 2011;16(4):458-66. doi: 10.1634/theoncologist.2010-0256. Epub 2011 Mar 4. Oncologist. 2011. PMID: 21378078 Free PMC article. Review.
Cited by
-
The management of cytologically indeterminate thyroid nodules in clinical practice: A contemporary perspective with focus on molecular imaging.Endocrine. 2025 Sep;89(3):710-716. doi: 10.1007/s12020-025-04299-4. Epub 2025 Jun 20. Endocrine. 2025. PMID: 40542314 Review.
-
Enhanced staging of differentiated thyroid carcinoma: integrating [18F]FDG digital PET/CT with neck ultrasound.Eur J Nucl Med Mol Imaging. 2025 Jul;52(8):2875-2886. doi: 10.1007/s00259-025-07169-3. Epub 2025 Feb 25. Eur J Nucl Med Mol Imaging. 2025. PMID: 39998675
-
Role of [18F]FDG PET/CT in the management of follicular cell-derived thyroid carcinoma.Cancer Imaging. 2024 Oct 28;24(1):147. doi: 10.1186/s40644-024-00791-8. Cancer Imaging. 2024. PMID: 39468677 Free PMC article. Review.
-
Thermal ablation for Bethesda III and IV thyroid nodules: current diagnosis and management.Ultrasonography. 2024 Nov;43(6):395-406. doi: 10.14366/usg.24083. Epub 2024 Aug 5. Ultrasonography. 2024. PMID: 39397446 Free PMC article.
-
Integrated Diagnostics of Thyroid Nodules.Cancers (Basel). 2024 Jan 11;16(2):311. doi: 10.3390/cancers16020311. Cancers (Basel). 2024. PMID: 38254799 Free PMC article. Review.
References
-
- Haugen BR, Alexander EK, Bible KC, et al. . 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26(1):1–133; doi: 10.1089/thy.2015.0020. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
