Tunnelling nanotube formation is driven by Eps8/IRSp53-dependent linear actin polymerization
- PMID: 38009333
- PMCID: PMC10711657
- DOI: 10.15252/embj.2023113761
Tunnelling nanotube formation is driven by Eps8/IRSp53-dependent linear actin polymerization
Abstract
Tunnelling nanotubes (TNTs) connect distant cells and mediate cargo transfer for intercellular communication in physiological and pathological contexts. How cells generate these actin-mediated protrusions to span lengths beyond those attainable by canonical filopodia remains unknown. Through a combination of micropatterning, microscopy, and optical tweezer-based approaches, we demonstrate that TNTs formed through the outward extension of actin achieve distances greater than the mean length of filopodia and that branched Arp2/3-dependent pathways attenuate the extent to which actin polymerizes in nanotubes, thus limiting their occurrence. Proteomic analysis using epidermal growth factor receptor kinase substrate 8 (Eps8) as a positive effector of TNTs showed that, upon Arp2/3 inhibition, proteins enhancing filament turnover and depolymerization were reduced and Eps8 instead exhibited heightened interactions with the inverted Bin/Amphiphysin/Rvs (I-BAR) domain protein IRSp53 that provides a direct connection with linear actin polymerases. Our data reveals how common protrusion players (Eps8 and IRSp53) form tunnelling nanotubes, and that when competing pathways overutilizing such proteins and monomeric actin in Arp2/3 networks are inhibited, processes promoting linear actin growth dominate to favour tunnelling nanotube formation.
Keywords: actin cytoskeleton; cell biophysics; proteomics; tunnelling nanotubes.
© 2023 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

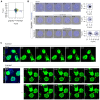
Schematic depicting fibronectin (FN) patterns produced as a hexagonal array of circles (diameter of 31 μm) with edge‐to‐edge separation distances (D) of 15, 20, 30, and 40 μm tuned along the centre‐to‐centre distance (d) between circles.
Left: Representative fluorescent images of FN micropatterns spaced by 15 and 40 μm with their respective calculated fast Fourier transform (FFT) images (upper right insets); Scale bars, 100 μm. Right: Corresponding radial profiles determined from the FFT images plotted as a function of the spatial frequency (μm−1). Solid lines in the radial profile plots indicate the expected centre‐to‐centre d spacing between circular patterns and line up nicely with peaks in the measured profile, indicating high fidelity in the manufacturing process.
Representative surface and upper stack maximum intensity projections of CAD cells plated on 15 μm FN micropatterns; Scale bars, 30 μm. TNTs connecting cells on two different micropatterns are annotated with yellow arrowheads. Subpanels (i, ii) show XZ projections made through the long axis of the indicated TNTs; Scale bars, 5 μm. Cells were fixed and stained with DAPI (blue), AX‐488 WGA (green) and AX‐647 Phalloidin (magenta); micropatterns were visualized using Rhodamine FN (red).
Representative surface and upper stack maximum intensity projections of CAD cells plated on 30 μm FN micropatterns; Scale bars, 30 μm. The yellow arrowhead indicates a TNT connecting cells on neighbouring micropatterns. Subpanels in i show an enlargement of the TNT‐connected cells (indicated by the yellow box) and XZ projections made through the long axis of the indicated TNT; Scale bars, 5 μm. Cells were fixed and stained with DAPI (blue), AX‐488 WGA (green) and AX‐647 Phalloidin (magenta); micropatterns were visualized using Rhodamine FN (red).
Representative maximum intensity projection of CAD cells plated on 40 μm FN micropatterns showing no TNTs connecting cells; Scale bar, 30 μm. Cells were fixed and stained with DAPI (blue), AX‐488 WGA (green) and AX‐647 Phalloidin (magenta); micropatterns were visualized using Rhodamine FN (red).
Violin plot of the percentage of TNT‐connected micropatterns for D15, D20, D30 and D40 micropatterns. The median percentage of TNT‐connected micropatterns (solid teal lines in the plot) on D15, D20, D30 and D40 micropatterns is 9.8%, 5.5%, 4.4%, and 0%, respectively; quartiles are annotated with dotted teal lines. Each data point corresponds to a quantified image in which on average approximately 30 cell‐occupied micropatterns were within the acquired field of view; data was pooled from three experiments. The total number of individual micropatterns quantified per condition is indicated in parentheses. Statistical analysis was performed using a Kruskal Wallis test with Dunn's multiple comparison test. Adjusted P values for each comparison are provided on the plot.
Left: Representative images of CAD cell filopodia (magenta, AX‐647 Phalloidin); Scale bars, 5 μm. Cell body edges are marked with a dotted white line. Right: Dot plot of individual filopodia lengths (18 cells, n = 548 filopodia) and the corresponding histogram showing that 90% of the population (shaded grey region) had lengths that were less than 6 μm.
- A, B
Assessment of CAD cell confinement over an extended period of time (18 h) on D15 fibronectin micropatterns. (A) Plot of individual cell trajectories normalized with respect to the centre of their micropattern (n = 56 trajectories). (B) Representative time‐lapse images of CAD cells and their corresponding trajectories. Fibronectin micropatterns are false‐coloured in blue (Rhodamine fibronectin). Scale bars, 10 μm.
- C
Selected time frames from a 30 min (Example 1, Movie EV5) and an overnight (Example 2, Movie EV6) acquisition showing TNT‐like protrusion formation between D15 micropatterned cells (expressing F‐Tractin, green) occurs through actin‐based protrusions. Images of the F‐Tractin channel are max intensity projections of the upper stacks in the acquired Z range and were overlayed with the fibronectin channel to highlight cell residency to the micropatterns. White dotted circles annotate the AX‐405‐labelled fibronectin patterns (blue) shown on the left, and yellow arrowheads point to representative TNTs. Scale bars, 10 μm.
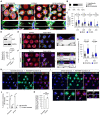
TNT‐connected cells characteristically lack lamellipodial features. Representative maximum intensity projection images of cells with different morphologies that are connected by TNTs; Scale bars, 30 μm. TNTs can be formed between: (i) two cells forming only lamellipodia, (ii) two cells showing a mix of phenotypes (e.g., one of the connected cells displays only (or partial) lamellipodia while the other connected cell shows no lamellipodia), or (iii) two non‐lamellipodia cells displaying a higher density of peripheral protrusions. Dashed yellow boxes correspond to subpanels below each image that show expanded views and XZ projections of TNTs that were made through the long axis of the connection; Scale bars, 5 μm. Yellow arrowheads directly point to the TNT.
Bar graph showing the characterization of TNTs between cells of different morphologies plated on D15 micropatterns (n = 97 TNTs). Lamellipodia, 4.1%; Mixed, 24.7%; and No Lamellipodia, 71.1%.
Whisker box plot showing the percentage of TNT‐connected micropatterns for D15, D20, D30 and D40 micropatterns in cells treated with 50 μM CK‐666 as compared to the mock condition (DMSO vehicle control). Mock vs. CK‐666 average values were 8.8 ± 1.5% vs. 15.5 ± 2.0% for D15 micropatterns, 5.0 ± 0.8% vs. 10.4 ± 1.3% for D20 micropatterns, 3.6 ± 0.7% vs. 8.5 ± 1.3% for D30 micropatterns, and 2.0 ± 0.9% vs. 2.3 ± 0.8% for D40 micropatterns (mean ± SEM). Each data point corresponds to a quantified image in which on average approximately 30 cell‐occupied micropatterns were within the acquired field of view. Representative images of the different conditions can be found in Appendix Fig S4. The total number of individual micropatterns quantified in each condition is indicated below each box. Data was pooled from 3 experiments and was analysed using an unpaired Mann–Whitney test.
siRNA knockdown of Actr3 (Arp3) in CAD cells. Top: Representative Western blot of Scramble control cells and siActr3 cells revealed with α‐Actr3 and α‐GAPDH (loading control) antibodies. Bottom: Graph showing the relative expression of Actr3 in Scramble control cells (set to 100%) and siActr3 cells (1.9 ± 1.2%, mean ± SEM). Statistical analysis was performed using an unpaired Student's t‐test (n = 10 biological repeats of the knockdown).
Representative surface and upper stack maximum intensity projections of Scramble control CAD cells plated on D15 micropattern; Scale bars, 30 μm. Subpanels in i show XZ projections made through the long axis of the indicated TNT (yellow arrowhead); Scale bars, 5 μm. Cells were fixed and stained with DAPI (blue), AX‐488 WGA (green), and AX‐647 Phalloidin (magenta); micropatterns were visualized using Rhodamine FN (red).
Representative surface and upper stack maximum intensity projections of siActr3 treated CAD cells plated on D15 micropatterns; Scale bars, 30 μm. Subpanels (i, ii) show XZ projections made through the long axis of the indicated TNTs (yellow arrowheads); Scale bars, 5 μm. Cells were fixed and stained with DAPI (blue), AX‐488 WGA (green) and AX‐647 Phalloidin (magenta); micropatterns were visualized using Rhodamine‐FN (red).
Whisker box plot showing the percentage of TNT‐connected micropatterns for Scramble control and siActr3 CAD cells plated on D15 and D30 micropatterns. Scramble vs. siActr3 average values were 5.9 ± 0.7% vs. 20.5 ± 1.9% for D15 micropatterns and 2.6 ± 0.6% vs. 6.3 ± 1.1% for D30 micropatterns (mean ± SEM). Each data point corresponds to a quantified image in which on average approximately 20 cell‐occupied micropatterns were within the acquired field of view. Representative images of the different conditions can be found in Appendix Fig S5. The total number of individual micropatterns quantified in each condition is indicated below each box. Data for D15 and D30 was pooled from 4 and 3 experiments, respectively, and was analysed using an unpaired Mann–Whitney test.
Representative images of Scramble‐treated donor cells (d) co‐cultured with H2B‐EBFP expressing acceptor cells (a) on D15 micropatterns; Scale bars, 30 μm. White arrowheads annotate donor‐originating DiD‐labelled vesicles (white) in an acceptor cell. The yellow box indicates the enlarged area shown in the upper right inset. Cells were fixed and stained with AX‐488 WGA (green) and Rhodamine Phalloidin (red); micropatterns were visualized using AX‐405 FN (blue).
Representative images of siActr3‐treated donor cells (d) co‐cultured with H2B‐EBFP expressing acceptor cells (a) on D15 micropatterns; Scale bars, 30 μm. Subpanels in i show an enlargement of the enclosed area noted by the yellow box in the main micrographs; Scale bars, 30 μm. White arrowheads annotate donor‐originating DiD‐labelled vesicles (white) in an acceptor cell; yellow arrowheads annotate TNTs connecting cells on neighbouring micropatterns. Indicated crosshairs (ii, iii) correspond to XZ and YZ orthogonal projections of internalized vesicles in the subpanels; Scale bars, 5 μm. Cells were fixed and stained with AX‐488 WGA (green) and Rhodamine Phalloidin (red); micropatterns were visualized using AX‐405 FN (blue).
Bar graph showing the percentage of H2B‐EBFP acceptors containing DiD‐labelled vesicles received through cell–cell contact (i.e., corrected for secretion‐based transfer) from Scramble‐ (24.9 ± 1.4%) and siActr3‐treated (30.9 ± 0.9%) donors. Corresponding secretion‐based transfer levels used for determining contact‐mediated transfer levels are shown for comparison and were 3.3 ± 0.4% and 1.1 ± 0.1% for Scramble and siActr3, respectively. The total number of quantified acceptor cells is indicated for each condition. Data are from three individual experiments and are represented as a mean ± SEM.
Bar graph showing the relative percentage of H2B‐EBFP acceptors containing DiD‐labelled vesicles from siActr3‐treated donors (124.5 ± 3.9%, mean ± SEM) as compared to Scramble control donors (set to 100%). Data are from three individual experiments and were analysed using an unpaired Student's t‐test.

- A
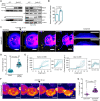
Experimental setup for pulling membrane nanotubes using a concanavalin A (Con‐A)‐coated bead (~3 μm in diameter) trapped in an optical tweezer (OT). CAD cells stably expressing the small F‐actin‐binding peptide F‐Tractin fused to EGFP (green) were exogenously labelled with the lipophilic Cell Mask™ Deep Red plasma membrane stain (magenta). Intensity profiles of the F‐actin fluorescence were measured along the nanotube axis and plotted against the nanotube length. Pulled nanotube lengths averaged 14.2 ± 0.6 μm (mean ± SEM, n = 23 tubes).
- B, C
Time‐lapse montages of nanotubes pulled from a DMSO‐treated cell (Movie EV7) and a CK‐666‐treated cell (Movie EV8). Displayed intensities for the F‐Tractin channel were set between 0 and 5 photon counts (cts) for better visualization. White arrowheads annotate the progression of actin development within the nanotube, while cyan arrowheads annotate obstructing protrusions that grew along the pulled nanotube. The trapped bead is annotated with a dotted white circle when not clearly visible. Scale bars, 5 μm.
- D, E
Corresponding kymographs for the time‐lapse montages in (B) and (C), generated along the nanotube axis (yellow dotted lines in B and C). Dotted white and cyan lines in the kymographs mark the cell body edge and obstructing protrusions, respectively. Scale bars, 5 μm.
- F
Plot of actin profiles within pulled nanotubes for mock‐ and CK‐666‐treated cells. Insets show a magnified view at the tube extremity to better highlight the greater presence of F‐actin in the CK‐666 condition as compared to the mock condition. Mock condition, 11 tubes; CK‐666 condition, 12 tubes.
- G
Left: Exponential fits to the actin intensity profiles were performed to determine a characteristic decay length (2ℓ) at which the initial intensity at X = 0 decays to a value of 1/e 2. For visualization purposes of the analysis, exponential fits are shown for the mean actin profiles computed from the individual plots presented in (F). Upper and lower limits of the intensity range are shaded. Right: Dot plot of the characteristic decay lengths (2ℓ) for mock‐ and CK‐666‐treated cells. Data is represented as the mean ± SEM. Mock (11 tubes), 2.78 ± 0.50; CK‐666 (12 tubes), 5.80 ± 0.73. Statistical analysis was performed using an unpaired Mann–Whitney test.
- H
Left: Force plot of a pulled nanotube from a mock‐treated cell showing no F‐actin development. Solid teal line, 10‐point moving average curve. Right: Associated images of the indicated time points (i, ii). The trapped bead is annotated with a dotted white circle when not clearly visible. Scale bars, 5 μm.
- I
Left: Force plot of a pulled nanotube from a CK‐666‐treated cell showing F‐actin development spanning the entire nanotube length. Peaks in the force plot (black arrowheads), with magnitudes of ΔF, arise when retrograde flows outcompete actin polymerization (at the nanotube tip) causing bead displacement towards the cell body (recorded as a positive rise in the force in the lab frame). Solid teal line, 10‐point moving average curve. Shaded grey region corresponds to a magnified view in the centre. Right: Associated images of the indicated time points (i, ii). The trapped bead is annotated with a dotted white circle when not clearly visible. Scale bars, 5 μm.
- J
Histogram of the force peak magnitudes (ΔF). Sample size, 33 peaks.

Representative nanotube pulled to an extreme length from a DMSO‐treated CAD cell showing little to no actin polymerization within. Displayed F‐Tractin intensities were set between 0 and 10 photon counts for better visualization. The trapped bead is annotated with a dotted white circle when not clearly visible. Scale bars, 5 μm.
Plot of actin profiles within pulled nanotubes of extreme lengths for DMSO‐treated CAD cells (n = 21 tubes). The solid green line is an exponential fit to the combined data.
Representative nanotube pulled to an extreme length from a CK‐666‐treated (50 μM) CAD cell showing actin growth 20 μm within the nanotube. Displayed F‐Tractin intensities were set between 0 and 10 photon counts for better visualization. The trapped bead is annotated with a dotted white circle when not clearly visible. Scale bars, 5 μm.
Plot of actin profiles within pulled nanotubes of extreme lengths for CK‐666‐treated CAD cells (n = 21 tubes). The solid green line is an exponential fit to the combined data.
Categorization of the percentage of pulled nanotubes with F‐actin profiles becoming indistinguishable from background intensity levels within a given micron range for DMSO‐ and CK‐666‐treated CAD cells.
Representative Western Blots showing GFP‐trap immunoprecipitation bands of the IRSp53 and IRTKS prey proteins in the GFP‐Eps8‐WT bait precipitate (left) and input lysate (right), with or without 50 μM CK‐666 treatment.
Relative IRSp53 band intensity quantification. Both the CK‐666‐treated and DMSO‐treated bands for IRSp53 were normalized with respect to their Eps8‐WT bait bands (detected by an anti‐GFP antibody). Relative band intensity was 157.5 ± 8.9% for the CK‐666 condition as compared to the DMSO control (was set to 100%). Data are from three individual experiments and are represented as a mean ± SEM. Statistical analysis was performed using a t‐test with Welch's correction, P = 0.0235.
Representative time‐lapse images (2, 36, 45, and 56 min from Movie EV9) of protrusion formation in IRSp53‐transfected cells before and after CK‐666 addition (at 30 min). Yellow arrowheads show protrusions that are formed after CK‐666 addition. Subpanels (i, ii) show the enlarged sections of the cell edge before (i) and after (ii) the addition of CK‐666, demonstrating the recruitment of IRSp53 at the membrane (ii). Scale bars, 10 μm.
Scatter plot comparing the maximum protrusion length before (4.3 ± 0.2 μm) and after CK‐666 addition (13.2 ± 0.3 μm) in IRSp53‐transfected cells containing the F‐actin label tdTomato‐F‐Tractin. Twenty‐five cells were analysed, and the number of protrusions analysed in each condition is indicated in parentheses. Data are from 10 individual experiments and are represented as a mean ± SEM. Statistical analysis was performed using an unpaired t‐test with Welch's correction, P < 0.0001.
Profile plots of IRSp53 intensity determined across the membrane before (left) and after the addition of CK‐666 (right). Magnified views of the cell edge (dashed black boxes) are provided to better highlight the recruitment of IRSp53 at the cell membrane in the CK‐666 condition as compared to the control. Two cells were analysed: before CK‐666, 28 measurements; after CK‐666, 25 measurements.
Representative time‐lapse images (0, 15, 45 and 65 min from Movie EV10) of protrusion formation in Eps8‐ΔCAP‐transfected cells before and after CK‐666 addition (at 30 min). Yellow arrowheads show protrusions that are formed after CK‐666 addition. Scale bars, 20 μm.
Scatter plot comparing the maximum protrusion length before (7.4 ± 0.3 μm) and after CK‐666 addition (15.7 ± 0.5 μm) in Eps8‐ΔCAP‐transfected cells containing the F‐actin label tdTomato‐F‐Tractin. Twenty‐one cells were analysed, and the number of protrusions analysed in each condition is indicated in parentheses. Data are from nine individual experiments and are represented as a mean ± SEM. Statistical analysis was performed using unpaired t‐test with Welch's correction and P value <0.0001.

Bar graph showing the quantification of TNT‐connected cells prior to immunoprecipitation for GFP + DMSO (297 cells analysed; 100%), GFP + CK‐666 (229 cells analysed; 144.0 ± 5.4%), GFP‐Eps8‐WT + DMSO (224 cells analysed; 129.4 ± 6.2%) and GFP‐Eps8‐WT + CK‐666 cells (246 cells analysed; 150.0 ± 9.5%). Data are from three individual experiments and are represented as a mean ± SEM. Statistical analysis was performed using an ordinary ANOVA with Tukey's multiple comparison test. P values for each comparison are stated on the bar graph.
Representative images of surface and upper stacks of mCherry‐transfected control cells and IRTKS‐mCherry transfected cells plated on nonpatterned surfaces. Yellow arrowheads annotate TNT‐like protrusions. Subpanels (i) show the XY and YZ projections through the axis of the TNT indicated in the dashed yellow box.
Bar graph showing quantification of TNT‐connected cells in mCherry control (309 cells analysed; 23.6 ± 4%) and in IRTKS‐mCherry cells (268 cells analysed; 26.5 ± 3.9%). Data are from three individual experiments and are represented as a mean ± SEM. Statistical analysis was performed using a t‐test with Welch's correction, P = 0.6248.
Left: Bar graph showing total transfer analysis in GFP control co‐culture (100%) and IRTKS‐GFP co‐culture (96.5 ± 1.3%). Data are from two individual experiments and are represented as a mean ± SEM. Right: Gating strategy for flow cytometry measurements of total transfer. Q2 represents H2B‐mCherry‐labelled acceptor cells containing donor‐derived DiD vesicles.
Left: Representative surface images of GFP:mCherry (control), GFP‐Eps8‐WT:IRTKS‐mCherry and GFP‐Eps8‐ΔCAP:IRTKS‐mCherry co‐transfected cells. Right: Representative images of upper stacks of GFP:mCherry, GFP‐Eps8‐WT:IRTKS‐mCherry and GFP‐Eps8‐ΔCAP:IRTKS‐mCherry co‐transfected cells. Yellow arrowheads annotate TNT‐like protrusions. Cells were plated on nonpatterned surfaces.
Bar graph showing the quantification of TNT‐connected cells in GFP:mCherry (453 cells analysed; 100%), Eps8‐WT:IRTKS (203 cells analysed; 36.0 ± 5.9%) and in Eps8‐ΔCAP:IRTKS (252 cells analysed; 45.1 ± 5.9%) co‐transfected control cells. Data are from three individual experiments and are represented as a mean ± SEM. Statistical analysis was performed using an ordinary ANOVA with Dunnett's multiple comparison test. P values for each comparison are stated on the bar graph.

Representative surface and upper stack images of control GFP:mCherry and GFP‐Eps8‐ΔCAP:IRSp53‐mCherry expressing CAD cells plated on D15 micropatterns (Alexa 405‐labelled FN) treated with either DMSO or CK‐666. Subpanels (i–iv) show magnified projections of the TNTs indicated in the dashed yellow boxes; XZ and YZ projections were made through the axis of the TNT.
Whisker box plot of the percentage of TNT‐connected cells on D15 micropatterns for GFP:mCherry + DMSO (10.3 ± 1.6%), Eps8‐ΔCAP:IRSp53 + DMSO (20.8 ± 2.8%), GFP:mCherry + CK‐666 (21.4 ± 3.1%), and Eps8‐ΔCAP:IRSp53 + CK‐666 cells (23.5 ± 2.4%) (mean ± SEM). Total number of quantified cells on patterns is indicated below the Whisker plots for each condition. Data were pooled from three individual experiments and each data point corresponds to a quantified image in which on average approximately 10 cell‐occupied micropatterns were within the acquired field of view. Mean values are indicated as a + symbol on the graph for each condition. Statistical analysis was performed using a Kruskal Wallis test with Dunn's multiple comparison test. Significant P values for each comparison are stated on the plot, ns = nonsignificant for P > 0.9999.
Representative images of GFP‐Eps8‐ΔCAP:IRSp53‐mCherry donor cells (d) co‐cultured with H2B‐EBFP acceptors (a) treated with either DMSO or CK‐666. Dashed yellow circles annotate Alexa 405‐labelled FN patterns for clarity and yellow arrowheads show the donor‐originating vesicle in the acceptor cell. Subpanels (i, ii) show magnified projections of the acceptor cell containing DiD‐labelled vesicles (yellow arrowheads); XZ and YZ projections were made through the vesicle.
Bar graph showing the percentage of H2B‐EBFP acceptors containing DiD‐stained vesicles in GFP:mCherry + DMSO (28.8 ± 1.7%), Eps8‐ΔCAP:IRSp53 + DMSO (35.7 ± 0.7%), GFP:mCherry + CK‐666 (37.0 ± 1.9%), and Eps8‐ΔCAP:IRSp53 + CK‐666 (37.2 ± 1.5%) experiments. Total number of quantified acceptor cells is indicated for each condition. Data are from at least three individual experiments and are represented as a mean ± SEM. Statistical analysis was performed using an ordinary ANOVA with Tukey's multiple comparison test. Significant P values for each comparison are stated on the bar graph. ns = nonsignificant for GFP:mCherry + CK‐666 vs. Eps8‐ΔCAP:IRSp53 + DMSO, P = 0.9246; for GFP:mCherry + CK‐666 vs. Eps8‐ΔCAP:IRSp53 + CK‐666, P = 0.9999; and for Eps8‐ΔCAP:IRSp53 + DMSO vs. Eps8‐ΔCAP:IRSp53 + CK‐666, P = 0.8807.
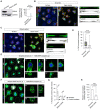
siRNA knockdown of IRsp53 in CAD cells. Left: Representative Western blot of Scramble control cells and IRSp53 siRNA cells revealed with α‐IRSp53 and α‐GAPDH (loading control) antibodies. Right: Graph showing the relative expression of IRSp53 in Scramble control cells (set to 100%) and IRSp53 siRNA cells (10.8 ± 8.7%, mean ± SEM). Statistical analysis was performed using an unpaired Student's t‐test (n = 3 biological repeats of the knockdown).
Representative surface and upper stack images of control CAD cells plated on D15 micropatterns treated with Scramble siRNA; Scale bars, 30 μm. Subpanels (i, ii) show XZ projections made through the long axis of the indicated TNTs (yellow arrowheads); Scale bars, 5 μm. Cells were fixed and stained with DAPI (blue), AX‐488 WGA (green) and Rhodamine Phalloidin (red); micropatterns were visualized using AX‐405 FN (blue).
Representative surface and upper stack images of IRSp53 knock‐down CAD cells plated on D15 micropatterns; Scale bars, 30 μm. Subpanels (i, ii) show XZ projections made through the long axis of the indicated TNTs (yellow arrowheads); Scale bars, 10 μm. Cells were fixed and stained with DAPI (blue), AX‐488 WGA (green) and Rhodamine Phalloidin (red); micropatterns were visualized using AX‐405 FN (blue).
Whisker box plot showing the percentage of TNT‐connected micropatterns for Scramble control and IRSp53 siRNA CAD cells plated on D15 micropatterns. Scramble vs. IRSp53 siRNA average values were 6.8 ± 0.8% vs. 0.7 ± 0.7% (mean ± SEM). Each data point corresponds to a quantified image in which on average approximately 10 cell‐occupied micropatterns were within the acquired field of view. The total number of individual micropatterns quantified in each condition is indicated below each box. Data was pooled from three experiments and was analysed using an unpaired Mann–Whitney test.
Representative images of Scramble‐treated donor cells (d) co‐cultured with H2B‐EBFP (blue) expressing acceptor cells (a) on D15 micropatterns; Scale bars, 20 μm. Indicated crosshairs (i, ii) correspond to XZ and YZ orthogonal projections of DiD‐labelled vesicles (white) internalized within acceptor cells (see subpanels); Scale bars, 10 μm. Cells were fixed and stained with AX‐488 WGA (green); micropatterns were unstained but visible upon over‐saturation in the green channel and outlined with dotted white circles for clarity.
Representative images of IRSp53 siRNA‐treated donor cells (d) co‐cultured with H2B‐EBFP (blue) expressing acceptor cells (a) on D15 micropatterns; Scale bars, 20 μm. The indicated crosshair (i) corresponds to XZ and YZ orthogonal projections of an example DiD‐labelled vesicle (white) internalized within an acceptor cell (see subpanels); Scale bars, 10 μm. Cells were fixed and stained with AX‐488 WGA (green); micropatterns were unstained but visible upon over‐saturation in the green channel and outlined with dotted white circles for clarity.
Bar graph showing the percentage of H2B‐EBFP acceptors containing DiD‐labelled vesicles received through cell–cell contact (i.e., corrected for secretion‐based transfer) from Scramble‐ (21.8 ± 5.8%) and IRSp53 siRNA‐treated (9.7 ± 3.4%) donors. Corresponding secretion‐based transfer levels used for determining contact‐mediated transfer levels are shown for comparison and were 0.9 ± 0.5% and 1.7 ± 0.9% for Scramble and siActr3, respectively. The total number of quantified acceptor cells is indicated for each condition. Data are from three individual experiments and are represented as a mean ± SEM.
Bar graph showing the relative percentage of H2B‐EBFP acceptors containing DiD‐labelled vesicles from IRSp53 siRNA‐treated donors (42.0 ± 4.5%, mean ± SEM) as compared to Scramble control donors (set to 100%). Data are from three individual experiments and were analysed using an unpaired Student's t‐test.

Selected time frames from Movie EV15 showing the duration of a TNT connecting EGFP F‐Tractin expressing control cells treated with DMSO. Displayed images of the F‐Tractin channel (false coloured in the “Fire” lookup table) are max intensity projections of the upper stacks in the acquired Z range. At the initial time point (left large panel), the D15 micropatterns (AX‐405 FN, blue) visualized at the sample surface are overlayed for reference. Yellow arrowheads point to the TNT throughout its lifetime. Scale bars, 20 μm (large panel) and 10 μm (subpanels).
Selected time frames from Movies EV16 and EV17 showing the duration of a TNT connecting iRFP670‐Eps8‐ΔCAP, IRSp53‐mCherry, and EGFP F‐Tractin expressing CAD cells treated with DMSO. Displayed images of the F‐Tractin channel (false coloured in the “Fire” lookup table) are max intensity projections of the upper stacks in the acquired Z range. At the initial time point, the D15 micropatterns (AX‐405 FN, blue) visualized at the sample surface are overlayed with the F‐Tractin channel and outlined with white dotted circles for reference (top large panel); additionally, images confirming Eps8 and IRSp53 expression are presented (bottom panels with inverted greyscales). Yellow arrowheads point to the TNT throughout its lifetime. Cyan dotted circles annotate an observed transfer event (see Movie EV17). Scale bars, 20 μm (large panel) and 10 μm (subpanels).
Selected time frames from Movie EV18 showing the duration of a TNT connecting EGFP F‐Tractin expressing control cells treated with 50 μM CK‐666. Displayed images of the F‐Tractin channel (false coloured in the “Fire” lookup table) are max intensity projections of the upper stacks in the acquired Z range. At the initial time point (left large panel), the D15 micropatterns (AX‐405 FN, blue) visualized at the sample surface are overlayed for reference. Yellow arrowheads point to the TNT throughout its lifetime. Yellow dotted lines annotate sections of the TNT with weak F‐Tractin fluorescence. Scale bars, 20 μm (large panel) and 10 μm (subpanels).
Selected time frames from Movie EV19 showing the duration of a TNT connecting i670‐Eps8‐ΔCAP, IRSp53‐mCherry, and EGFP F‐Tractin expressing CAD cells treated with 50 μM CK‐666. Displayed images of the F‐Tractin channel (false coloured in the “Fire” lookup table) are max intensity projections of the upper stacks in the acquired Z range. At the initial time point, the D15 micropatterns (AX‐405 FN, blue) visualized at the sample surface are overlayed with the F‐Tractin channel and outlined with white dotted circles for reference (top large panel); additionally, images confirming Eps8 and IRSp53 expression are presented (bottom panels with inverted greyscales). Yellow arrowheads point to the TNT throughout its lifetime. Yellow dotted lines annotate sections of the TNT with weak F‐Tractin fluorescence. Scale bars, 20 μm (large panel) and 10 μm (subpanels).
Violin plot of TNT durations for control cells (those only expressing EGFP F‐Tractin) and cells additionally co‐expressing i670‐Eps8‐ΔCAP and IRSp53‐mCherry mock treated with DMSO or treated with 50 μM CK‐666. Median TNT durations were: Ctrl + DMSO, 33.50 min (n = 19); Eps8‐ΔCAP:IRSp53 + DMSO, 66.50 min (n = 21); Ctrl + CK‐666, 36.13 min (n = 18); and Eps8‐ΔCAP:IRSp53 + CK‐666, 64.75 min (n = 23). The black dotted line marks TNTs remaining up until the maximum allotted observational time. Statistical analysis was performed using a Kruskal Wallis test with Dunn's multiple comparison test. Adjusted P values for each comparison are provided on the plot.

- A
Schematic depicting the GFP‐Trap immunoprecipitation strategy for identifying actin‐related protein hits associated with Eps8 and IRSp53 “bait” proteins.
- B, C
STRING‐generated networks of differentially abundant proteins present in Eps8‐WT as compared to the negative control for nontreated (DMSO) (B) and CK‐666‐treated (C) CAD cells. The sizes of the nodes (i.e., protein gene names) were scaled based on the degree of connectivity in the networks, and the transparency of the edges relate to the calculated score of the protein–protein interaction in the STRING database.
- D
Direct comparison of identified protein hits between nontreated and treated Eps8 samples. Left: iBAQ plot of proteins only present in the Eps8 treated sample. Centre: Volcano plot of common proteins to both nontreated and treated Eps8 samples. Dashed vertical lines mark the binary logarithm position of a fold change of 1.5, and the dashed horizontal lines marks the adjusted P‐value threshold. Cyan‐shaded square corresponds to a magnified view of the indicated region on the right of the volcano plot. Right: iBAQ plot of proteins only present in the Eps8 nontreated sample.

Left: Proportion of protein hits for a given GO term. The number of protein hits in the network for a given GO term was normalized by the total number of proteins assigned to a given GO term using the whole mouse genome as a reference (Ref count in B). The graph is coloured by the false discovery rate (FDR). Sizes reflect the fold enrichment of the number proteins in the network divided by the number of proteins expected to be annotated with a given GO term in a randomly generated network of the same size. Right: Relative change in the proportion of proteins in a GO term when comparing CK‐666‐treated to nontreated Eps8‐WT expressing CAD cells.
Table summarizing mapped proteins to their corresponding GO term for (A). The number of reference proteins in the mouse genome for a given GO term is provided (Ref count). Colour code: Teal, proteins only present in the nontreated (DMSO) Eps8 pull down; Magenta, proteins only present in the CK‐666‐treated Eps8 pull down; Black, proteins common to both pull‐downs.
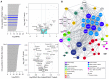
Direct comparison between nontreated and treated IRSp53 samples. Left: iBAQ plot of proteins only present in the treated (top) and nontreated (bottom) IRSp53 samples. Right: Volcano plots of common proteins to both nontreated and treated IRSp53 samples. Dashed vertical lines mark the binary logarithm position of a fold change of 1.5, and the dashed horizontal line marks the adjusted P‐value threshold. Cyan‐shaded square corresponds to a magnified view of the indicated region.
Combined STRING‐generated network of all identified protein hits in the IRSp53 pull down (both nontreated and treated samples). The sizes of the nodes (i.e., protein gene names) were scaled based on the degree of connectivity in the networks, and the transparency of the edges relate to the calculated score of the protein–protein interaction in the STRING database. Italicized hits are those proteins present only in the treated (e.g., Gsn, Arpc1b, Arpc3, Tmod3, Rab10, Myh3) or nontreated (e.g., Sptbn5) IRSp53 sample shown in (A).
References
-
- Abella JVG, Galloni C, Pernier J, Barry DJ, Kjær S, Carlier M‐F, Way M (2016) Isoform diversity in the Arp2/3 complex determines actin filament dynamics. Nat Cell Biol 18: 76–86 - PubMed
-
- Alarcon‐Martinez L, Villafranca‐Baughman D, Quintero H, Kacerovsky JB, Dotigny F, Murai KK, Prat A, Drapeau P, Di Polo A (2020) Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature 585: 91–95 - PubMed
-
- Almagro S, Durmort C, Chervin‐Pétinot A, Heyraud S, Dubois M, Lambert O, Maillefaud C, Hewat E, Schaal JP, Huber P et al (2010) The motor protein myosin‐X transports VE‐cadherin along filopodia to allow the formation of early endothelial cell‐cell contacts. Mol Cell Biol 30: 1703–1717 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

