Engineered Probiotic-Based Personalized Cancer Vaccine Potentiates Antitumor Immunity through Initiating Trained Immunity
- PMID: 38009498
- PMCID: PMC10797439
- DOI: 10.1002/advs.202305081
Engineered Probiotic-Based Personalized Cancer Vaccine Potentiates Antitumor Immunity through Initiating Trained Immunity
Abstract
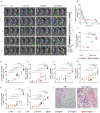
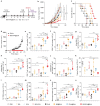
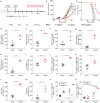
Cancer vaccines hold great potential for clinical cancer treatment by eliciting T cell-mediated immunity. However, the limited numbers of antigen-presenting cells (APCs) at the injection sites, the insufficient tumor antigen phagocytosis by APCs, and the presence of a strong tumor immunosuppressive microenvironment severely compromise the efficacy of cancer vaccines. Trained innate immunity may promote tumor antigen-specific adaptive immunity. Here, a personalized cancer vaccine is developed by engineering the inactivated probiotic Escherichia coli Nissle 1917 to load tumor antigens and β-glucan, a trained immunity inducer. After subcutaneous injection, the cancer vaccine delivering model antigen OVA (BG/OVA@EcN) is highly accumulated and phagocytosed by macrophages at the injection sites to induce trained immunity. The trained macrophages may recruit dendritic cells (DCs) to facilitate BG/OVA@EcN phagocytosis and the subsequent DC maturation and T cell activation. In addition, BG/OVA@EcN remarkably enhances the circulating trained monocytes/macrophages, promoting differentiation into M1-like macrophages in tumor tissues. BG/OVA@EcN generates strong prophylactic and therapeutic efficacy to inhibit tumor growth by inducing potent adaptive antitumor immunity and long-term immune memory. Importantly, the cancer vaccine delivering autologous tumor antigens efficiently prevents postoperative tumor recurrence. This platform offers a facile translatable strategy to efficiently integrate trained immunity and adaptive immunity for personalized cancer immunotherapy.
Keywords: antitumor immunity; cancer vaccines; probiotics; trained immunity; β-glucan.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Engineered mitochondria exert potent antitumor immunity as a cancer vaccine platform.Cell Mol Immunol. 2024 Nov;21(11):1251-1265. doi: 10.1038/s41423-024-01203-4. Epub 2024 Aug 20. Cell Mol Immunol. 2024. PMID: 39164536 Free PMC article.
-
Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer.Mol Cancer. 2022 Feb 11;21(1):45. doi: 10.1186/s12943-022-01515-x. Mol Cancer. 2022. PMID: 35148751 Free PMC article.
-
A spontaneous multifunctional hydrogel vaccine amplifies the innate immune response to launch a powerful antitumor adaptive immune response.Theranostics. 2021 May 8;11(14):6936-6949. doi: 10.7150/thno.58173. eCollection 2021. Theranostics. 2021. PMID: 34093863 Free PMC article.
-
Dendritic cell biology and its role in tumor immunotherapy.J Hematol Oncol. 2020 Aug 3;13(1):107. doi: 10.1186/s13045-020-00939-6. J Hematol Oncol. 2020. PMID: 32746880 Free PMC article. Review.
-
Dendritic Cells and Cancer Immunity.Trends Immunol. 2016 Dec;37(12):855-865. doi: 10.1016/j.it.2016.09.006. Epub 2016 Oct 25. Trends Immunol. 2016. PMID: 27793569 Free PMC article. Review.
Cited by
-
Trained immunity inducers in cancer immunotherapy.Front Immunol. 2024 Jul 16;15:1427443. doi: 10.3389/fimmu.2024.1427443. eCollection 2024. Front Immunol. 2024. PMID: 39081326 Free PMC article. Review.
-
Probiotic-Vaccine Synergy in Fish Aquaculture: Exploring Microbiome-Immune Interactions for Enhanced Vaccine Efficacy.Biology (Basel). 2025 May 29;14(6):629. doi: 10.3390/biology14060629. Biology (Basel). 2025. PMID: 40563880 Free PMC article. Review.
-
Multifaceted roles of trained immunity in diverse pathological contexts.BMB Rep. 2024 Oct;57(10):431-440. doi: 10.5483/BMBRep.2024-0045. BMB Rep. 2024. PMID: 38835118 Free PMC article. Review.
-
Morphology- and adhesion-dual biomimetic nanovaccine boosts antigen cross-presentation through subcellular transport regulation.Sci Adv. 2025 Aug;11(31):eadx6732. doi: 10.1126/sciadv.adx6732. Epub 2025 Jul 30. Sci Adv. 2025. PMID: 40737412 Free PMC article.
-
Nanotherapeutics for Macrophage Network Modulation in Tumor Microenvironments: Targets and Tools.Int J Nanomedicine. 2024 Dec 19;19:13615-13651. doi: 10.2147/IJN.S491573. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39717515 Free PMC article. Review.
References
-
- a) Saxena M., Van Der Burg S. H., Melief C. J. M., Bhardwaj N., Nat. Rev. Cancer 2021, 21, 360; - PubMed
- b) Briquez P. S., Hauert S., De Titta A., Gray L. T., Alpar A. T., Swartz M. A., Hubbell J. A., Front Bioeng Biotechnol 2020, 8, 19; - PMC - PubMed
- c) Lin M. J., Svensson‐Arvelund J., Lubitz G. S., Marabelle A., Melero I., Brown B D., Brody J. D., Nat Cancer 2022, 3, 911; - PubMed
- d) Zhang R., Billingsley M. M., Mitchell M. J., J. Controlled Release 2018, 292, 256. - PMC - PubMed
-
- a) Hao H., Wu S., Lin J., Zheng Z., Zhou Y., Zhang Y., Guo Q., Tian F., Zhao M., Chen Y., Xu X., Hou L., Wang X., Tang R., Nat. Biomed. Eng. 2023, 7, 928; - PubMed
- b) Bencherif S. A., Warren Sands R., Ali O. A., Li W. A., Lewin S. A., Braschler T. M., Shih T.‐Y., Verbeke C. S., Bhatta D., Dranoff G., Mooney D J., Nat. Commun. 2015, 6, 7556; - PMC - PubMed
- c) Nguyen T. L., Yin Y., Choi Y., Jeong J. H., Kim J., ACS Nano 2020, 14, 11623. - PubMed
-
- a) Xie X., Song T., Feng Y, Zhang H., Yang G., Wu C., You F., Liu Y., Yang H., Chem. Eng. J. 2022, 437, 135505;
- b) Wang J., Mamuti M., Wang H., ACS Biomater. Sci. Eng. 2020, 6, 6036. - PubMed
-
- a) Jiang J., Mei J., Yi S., Feng C., Ma Y., Liu Y., Liu Y., Chen C., Adv. Drug Delivery Rev. 2022, 180, 114046; - PubMed
- b) Wei Z., Zhang X., Yong T., Bie N., Zhan G., Li X., Liang Q., Li J., Yu J., Huang G., Yan Y., Zhang Z., Zhang B., Gan Lu, Huang Bo, Yang X., Nat. Commun. 2021, 12, 440; - PMC - PubMed
- c) Xu J., Ma Q., Zhang Y., Fei Z., Sun Y., Fan Q., Liu B., Bai J., Yu Y., Chu J., Chen J., Wang C., Nat. Commun. 2022, 13, 110. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
