Engineered Probiotic-Based Personalized Cancer Vaccine Potentiates Antitumor Immunity through Initiating Trained Immunity
- PMID: 38009498
- PMCID: PMC10797439
- DOI: 10.1002/advs.202305081
Engineered Probiotic-Based Personalized Cancer Vaccine Potentiates Antitumor Immunity through Initiating Trained Immunity
Abstract
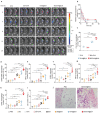
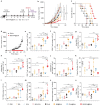
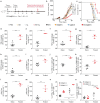
Cancer vaccines hold great potential for clinical cancer treatment by eliciting T cell-mediated immunity. However, the limited numbers of antigen-presenting cells (APCs) at the injection sites, the insufficient tumor antigen phagocytosis by APCs, and the presence of a strong tumor immunosuppressive microenvironment severely compromise the efficacy of cancer vaccines. Trained innate immunity may promote tumor antigen-specific adaptive immunity. Here, a personalized cancer vaccine is developed by engineering the inactivated probiotic Escherichia coli Nissle 1917 to load tumor antigens and β-glucan, a trained immunity inducer. After subcutaneous injection, the cancer vaccine delivering model antigen OVA (BG/OVA@EcN) is highly accumulated and phagocytosed by macrophages at the injection sites to induce trained immunity. The trained macrophages may recruit dendritic cells (DCs) to facilitate BG/OVA@EcN phagocytosis and the subsequent DC maturation and T cell activation. In addition, BG/OVA@EcN remarkably enhances the circulating trained monocytes/macrophages, promoting differentiation into M1-like macrophages in tumor tissues. BG/OVA@EcN generates strong prophylactic and therapeutic efficacy to inhibit tumor growth by inducing potent adaptive antitumor immunity and long-term immune memory. Importantly, the cancer vaccine delivering autologous tumor antigens efficiently prevents postoperative tumor recurrence. This platform offers a facile translatable strategy to efficiently integrate trained immunity and adaptive immunity for personalized cancer immunotherapy.
Keywords: antitumor immunity; cancer vaccines; probiotics; trained immunity; β-glucan.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- a) Saxena M., Van Der Burg S. H., Melief C. J. M., Bhardwaj N., Nat. Rev. Cancer 2021, 21, 360; - PubMed
- b) Briquez P. S., Hauert S., De Titta A., Gray L. T., Alpar A. T., Swartz M. A., Hubbell J. A., Front Bioeng Biotechnol 2020, 8, 19; - PMC - PubMed
- c) Lin M. J., Svensson‐Arvelund J., Lubitz G. S., Marabelle A., Melero I., Brown B D., Brody J. D., Nat Cancer 2022, 3, 911; - PubMed
- d) Zhang R., Billingsley M. M., Mitchell M. J., J. Controlled Release 2018, 292, 256. - PMC - PubMed
-
- a) Hao H., Wu S., Lin J., Zheng Z., Zhou Y., Zhang Y., Guo Q., Tian F., Zhao M., Chen Y., Xu X., Hou L., Wang X., Tang R., Nat. Biomed. Eng. 2023, 7, 928; - PubMed
- b) Bencherif S. A., Warren Sands R., Ali O. A., Li W. A., Lewin S. A., Braschler T. M., Shih T.‐Y., Verbeke C. S., Bhatta D., Dranoff G., Mooney D J., Nat. Commun. 2015, 6, 7556; - PMC - PubMed
- c) Nguyen T. L., Yin Y., Choi Y., Jeong J. H., Kim J., ACS Nano 2020, 14, 11623. - PubMed
-
- a) Xie X., Song T., Feng Y, Zhang H., Yang G., Wu C., You F., Liu Y., Yang H., Chem. Eng. J. 2022, 437, 135505;
- b) Wang J., Mamuti M., Wang H., ACS Biomater. Sci. Eng. 2020, 6, 6036. - PubMed
-
- a) Jiang J., Mei J., Yi S., Feng C., Ma Y., Liu Y., Liu Y., Chen C., Adv. Drug Delivery Rev. 2022, 180, 114046; - PubMed
- b) Wei Z., Zhang X., Yong T., Bie N., Zhan G., Li X., Liang Q., Li J., Yu J., Huang G., Yan Y., Zhang Z., Zhang B., Gan Lu, Huang Bo, Yang X., Nat. Commun. 2021, 12, 440; - PMC - PubMed
- c) Xu J., Ma Q., Zhang Y., Fei Z., Sun Y., Fan Q., Liu B., Bai J., Yu Y., Chu J., Chen J., Wang C., Nat. Commun. 2022, 13, 110. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
