HIF-1α-Induced Mitophagy Regulates the Regenerative Outcomes of Stem Cells in Fat Transplantation
- PMID: 38009534
- PMCID: PMC10683376
- DOI: 10.1177/09636897231210750
HIF-1α-Induced Mitophagy Regulates the Regenerative Outcomes of Stem Cells in Fat Transplantation
Abstract
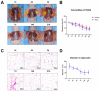
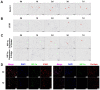
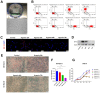
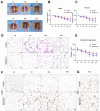
Hypoxia is a crucial factor with type diversity that plays an important role in stem cell transplantation. However, the effects of hypoxia on adipose-derived stem cells (ADSCs) are largely unclear in the autologous fat transplantation (AFT) model, which shows a special type of "acute-progressively resolving hypoxia." Here, an AFT model in nude mice and a hypoxic culture model for ADSCs were combined to explore the link between hypoxia-inducible factor-1 α subunit (HIF-1α) and mitophagy under hypoxic conditions. The results showed that the activity of ADSCs in the first 7 days after grafting was the key stage for volume retention, and the expression of HIF-1α, light chain 3 beta (LC3B), and Beclin1 in ADSCs increased during this period. We also found that hypoxia for longer than 48 h damaged the differentiation and mitochondrial respiration of ADSCs in vitro, but hypoxia signals also activate HIF-1α to initiate mitophagy and maintain the activities of ADSCs. Pre-enhancing mitophagy by rapamycin effectively improves mitochondrial respiration in ADSCs after grafting and ultimately improves AFT outcomes.
Keywords: HIF-1α; adipose-derived stem cells; autologous fat transplantation; cell transplantation; hypoxia; mitophagy.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




Similar articles
-
Adipose-derived mesenchymal stem cells with hypoxic preconditioning improve tenogenic differentiation.J Orthop Surg Res. 2022 Jan 28;17(1):49. doi: 10.1186/s13018-021-02908-2. J Orthop Surg Res. 2022. PMID: 35090498 Free PMC article.
-
Fascia-derived stem cells enhance fat graft retention by promoting vascularization through the HMOX1-HIF-1α pathway.Stem Cell Res Ther. 2025 Feb 25;16(1):92. doi: 10.1186/s13287-025-04204-w. Stem Cell Res Ther. 2025. PMID: 40001185 Free PMC article.
-
FSH prevents porcine granulosa cells from hypoxia-induced apoptosis via activating mitophagy through the HIF-1α-PINK1-Parkin pathway.FASEB J. 2020 Mar;34(3):3631-3645. doi: 10.1096/fj.201901808RRR. Epub 2020 Jan 20. FASEB J. 2020. PMID: 31960530
-
Hypoxic Regulation of Mitochondrial Metabolism and Mitophagy in Nucleus Pulposus Cells Is Dependent on HIF-1α-BNIP3 Axis.J Bone Miner Res. 2020 Aug;35(8):1504-1524. doi: 10.1002/jbmr.4019. Epub 2020 May 15. J Bone Miner Res. 2020. PMID: 32251541 Free PMC article.
-
Adipose-derived stem cells improve neovascularization in ischemic flaps in diabetic mellitus through HIF-1α/VEGF pathway.Eur Rev Med Pharmacol Sci. 2018 Jan;22(1):10-16. doi: 10.26355/eurrev_201801_14094. Eur Rev Med Pharmacol Sci. 2018. PMID: 29364466
Cited by
-
Applications of Engineered Skin Tissue for Cosmetic Component and Toxicology Detection.Cell Transplant. 2024 Jan-Dec;33:9636897241235464. doi: 10.1177/09636897241235464. Cell Transplant. 2024. PMID: 38491929 Free PMC article. Review.
References
-
- Trotzier C, Sequeira I, Auxenfans C, Mojallal AA. Fat graft retention: adipose tissue, adipose-derived stem cells, and aging. Plast Reconstr Surg. 2023;151(3):420e–31e. - PubMed
-
- Gerth DJ, King B, Rabach L, Glasgold RA, Glasgold MJ. Long-term volumetric retention of autologous fat grafting processed with closed-membrane filtration. Aesthet Surg J. 2014;34(7):985–94. - PubMed
-
- Mashiko T, Yoshimura K. How does fat survive and remodel after grafting? Clin Plast Surg. 2015;42(2):181–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

