Phenotyping of Macrophages After Radiolabeling and Safety of Intra-arterial Transplantation Assessed by SPECT/CT and MRI
- PMID: 38009543
- PMCID: PMC10683405
- DOI: 10.1177/09636897231212780
Phenotyping of Macrophages After Radiolabeling and Safety of Intra-arterial Transplantation Assessed by SPECT/CT and MRI
Abstract
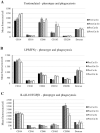
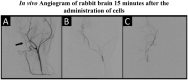
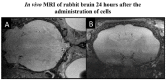
Cell therapy is an integral modality of regenerative medicine. Macrophages are known for their sensitivity to activation stimuli and capability to recruit other immune cells to the sites of injury and healing. In addition, the route of administration can impact engraftment and efficacy of cell therapy, and modern neuro-interventional techniques provide the possibility for selective intra-arterial (IA) delivery to the central nervous system (CNS) with very low risk. The effects of radiolabelling and catheter transport on differentially activated macrophages were evaluated. Furthermore, the safety of selective IA administration of these macrophages to the rabbit brain was assessed by single-photon emission computed tomography/computed tomography (SPECT/CT) and ultra-high-field (9.4 T) magnetic resonance imaging (MRI). Cells were successfully labeled with (111In)In-(oxinate)3 and passed through a microcatheter with preserved phenotype. No cells were retained in the healthy rabbit brain after IA administration, and no adverse events could be observed either 1 h (n = 6) or 24 h (n = 2) after cell administration. The procedure affected both lipopolysaccharide/gamma interferon (LPS/IFNγ) activated cells and interleukin 4 (IL4), interleukin 10 (IL10)/transforming growth factor beta 1 (TGFβ1) activated cells to some degree. The LPS/IFNγ activated cells had a significant increase in their phagocytotic function. Overall, the major impact on the cell phenotypes was due to the radiolabeling and not passage through the catheter. Unstimulated cells were substantially affected by both radiolabeling and catheter administration and are hence not suited for this procedure, while both activated macrophages retained their initial phenotypes. In conclusion, activated macrophages are suitable candidates for targeted IA administration without adverse effects on normal, healthy brain parenchyma.
Keywords: MRI; SPECT/CT; endovascular; intra-arterial; rabbit.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Safety of Intra-Arterial Injection With Tumor-Activated T Cells to the Rabbit Brain Evaluated by MRI and SPECT/CT.Cell Transplant. 2017 Feb 16;26(2):283-292. doi: 10.3727/096368916X693347. Epub 2016 Oct 7. Cell Transplant. 2017. PMID: 27725029 Free PMC article.
-
Image-Based Analysis of Tumor Localization After Intra-Arterial Delivery of Technetium-99m-Labeled SPIO Using SPECT/CT and MRI.Mol Imaging. 2017 Jan 1;16:1536012116689001. doi: 10.1177/1536012116689001. Mol Imaging. 2017. PMID: 28654377 Free PMC article.
-
Bone and Gallium Single-Photon Emission Computed Tomography-Computed Tomography is Equivalent to Magnetic Resonance Imaging in the Diagnosis of Infectious Spondylodiscitis: A Retrospective Study.Can Assoc Radiol J. 2017 Feb;68(1):41-46. doi: 10.1016/j.carj.2016.02.003. Epub 2016 Aug 11. Can Assoc Radiol J. 2017. PMID: 27523445
-
The role of single-photon emission computed tomography/computed tomography in benign and malignant bone disease.Semin Nucl Med. 2006 Oct;36(4):286-94. doi: 10.1053/j.semnuclmed.2006.05.001. Semin Nucl Med. 2006. PMID: 16950146 Review.
-
Single-photon emission computed tomography/computed tomography in brain tumors.Semin Nucl Med. 2007 Jan;37(1):34-47. doi: 10.1053/j.semnuclmed.2006.08.003. Semin Nucl Med. 2007. PMID: 17161038 Review.
References
-
- Lukawska JJ, Livieratos L, Sawyer BM, Lee T, O’Doherty M, Blower PJ, Kofi M, Ballinger JR, Corrigan CJ, Gnanasegaran G, Sharif-Paghaleh E, et al.. Real-time differential tracking of human neutrophil and eosinophil migration in vivo. J Allergy Clin Immunol. 2014;133(1). https://pubmed-ncbi-nlm-nih-gov.proxy.kib.ki.se/23953710/ [accessed 2023 Aug 21]. - PubMed
-
- Puncher MRB, Blower PJ. Autoradiography and density gradient separation of technetium-99m-exametazime (HMPAO) labelled leucocytes reveals selectivity for eosinophils. Eur J Nucl Med. 1994;21(11):1175–82. https://pubmed-ncbi-nlm-nih-gov.proxy.kib.ki.se/7859768/ [accessed 2023 Aug 21]. - PubMed
-
- Tavaré R, Sagoo P, Varama G, Tanriver Y, Warely A, Diebold SS, Southworth R, Schaeffter T, Lechler RI, Razavi R, Lombardi G, et al.. Monitoring of in vivo function of superparamagnetic iron oxide labelled murine dendritic cells during anti-tumour vaccination. Plos One. 2011;6(5). https://pubmed-ncbi-nlm-nih-gov.proxy.kib.ki.se/21637760/ [accessed 2023 Aug 21]. - PMC - PubMed
-
- Barrett AJ, Battiwalla M. Relapse after allogeneic stem cell transplantation. Expert Rev Hematol. 2010;3(4):429–41. https://pubmed-ncbi-nlm-nih-gov.proxy.kib.ki.se/21083034/ [accessed 2022 Mar 31]. - PMC - PubMed
-
- Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med. 2008;3(1):1–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

