Microstructural differences in the osteochondral unit of terrestrial and aquatic mammals
- PMID: 38009703
- PMCID: PMC10781421
- DOI: 10.7554/eLife.80936
Microstructural differences in the osteochondral unit of terrestrial and aquatic mammals
Abstract
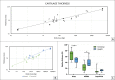
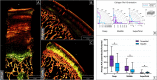
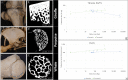
During evolution, animals have returned from land to water, adapting with morphological modifications to life in an aquatic environment. We compared the osteochondral units of the humeral head of marine and terrestrial mammals across species spanning a wide range of body weights, focusing on microstructural organization and biomechanical performance. Aquatic mammals feature cartilage with essentially random collagen fiber configuration, lacking the depth-dependent, arcade-like organization characteristic of terrestrial mammalian species. They have a less stiff articular cartilage at equilibrium with a significantly lower peak modulus, and at the osteochondral interface do not have a calcified cartilage layer, displaying only a thin, highly porous subchondral bone plate. This totally different constitution of the osteochondral unit in aquatic mammals reflects that accommodation of loading is the primordial function of the osteochondral unit. Recognizing the crucial importance of the microarchitecture-function relationship is pivotal for understanding articular biology and, hence, for the development of durable functional regenerative approaches for treatment of joint damage, which are thus far lacking.
Keywords: bone; cartilage; developmental biology; dolphin; mammals; regenerative medicine; stem cells; whale.
© 2023, Mancini et al.
Conflict of interest statement
IM, RL, MK, MC, MC, Mv, LI, MK, Pv, JM No competing interests declared
Figures







Update of
- doi: 10.1101/2022.06.09.495440
Similar articles
-
Effects of body mass on microstructural features of the osteochondral unit: A comparative analysis of 37 mammalian species.Bone. 2019 Oct;127:664-673. doi: 10.1016/j.bone.2019.07.001. Epub 2019 Jul 3. Bone. 2019. PMID: 31279095
-
Treatment of osteochondral defects in the rabbit's knee joint by implantation of allogeneic mesenchymal stem cells in fibrin clots.J Vis Exp. 2013 May 21;(75):e4423. doi: 10.3791/4423. J Vis Exp. 2013. PMID: 23728213 Free PMC article.
-
Subchondral bone plate thickness is associated with micromechanical and microstructural changes in the bovine patella osteochondral junction with different levels of cartilage degeneration.J Mech Behav Biomed Mater. 2022 May;129:105158. doi: 10.1016/j.jmbbm.2022.105158. Epub 2022 Mar 1. J Mech Behav Biomed Mater. 2022. PMID: 35279448
-
Role of the Osteochondral Unit in the Pathogenesis of Osteoarthritis: Focus on the Potential Use of Clodronate.Curr Rheumatol Rev. 2022;18(1):2-11. doi: 10.2174/1573397117666211006094117. Curr Rheumatol Rev. 2022. PMID: 34615451 Review.
-
Biomechanical Aspects of Osteochondral Regeneration: Implications and Strategies for Three-Dimensional Bioprinting.Tissue Eng Part B Rev. 2022 Aug;28(4):766-788. doi: 10.1089/ten.TEB.2021.0101. Epub 2021 Nov 2. Tissue Eng Part B Rev. 2022. PMID: 34409874 Free PMC article. Review.
Cited by
-
Restoring articular cartilage: insights from structure, composition and development.Nat Rev Rheumatol. 2025 May;21(5):291-308. doi: 10.1038/s41584-025-01236-7. Epub 2025 Mar 28. Nat Rev Rheumatol. 2025. PMID: 40155694 Review.
References
-
- Arokoski JP, Hyttinen MM, Lapveteläinen T, Takács P, Kosztáczky B, Módis L, Kovanen V, Helminen H. Decreased birefringence of the superficial zone collagen network in the canine knee (stifle) articular cartilage after long distance running training, detected by quantitative polarised light microscopy. Annals of the Rheumatic Diseases. 1996;55:253–264. doi: 10.1136/ard.55.4.253. - DOI - PMC - PubMed
-
- Benninghoff A. Form und bau der gelenkknorpel in ihren beziehungen zur funktion. Zeitschrift Für Anatomie Und Entwicklungsgeschichte. 1925;76:43–63. doi: 10.1007/BF02134417. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

