Gallionellaceae in rice root plaque: metabolic roles in iron oxidation, nutrient cycling, and plant interactions
- PMID: 38009924
- PMCID: PMC10734482
- DOI: 10.1128/aem.00570-23
Gallionellaceae in rice root plaque: metabolic roles in iron oxidation, nutrient cycling, and plant interactions
Abstract
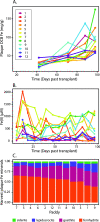
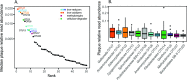
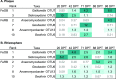
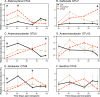
In waterlogged soils, iron plaque forms a reactive barrier between the root and soil, collecting phosphate and metals such as arsenic and cadmium. It is well established that iron-reducing bacteria solubilize iron, releasing these associated elements. In contrast, microbial roles in plaque formation have not been clear. Here, we show that there is a substantial population of iron oxidizers in plaque, and furthermore, that these organisms (Sideroxydans and Gallionella) are distinguished by genes for plant colonization and nutrient fixation. Our results suggest that iron-oxidizing and iron-reducing bacteria form and remodel iron plaque, making it a dynamic system that represents both a temporary sink for elements (P, As, Cd, C, etc.) as well as a source. In contrast to abiotic iron oxidation, microbial iron oxidation results in coupled Fe-C-N cycling, as well as microbe-microbe and microbe-plant ecological interactions that need to be considered in soil biogeochemistry, ecosystem dynamics, and crop management.
Keywords: iron oxyhydroxides; iron-oxidizing bacteria; iron-reducing bacteria; rice rhizosphere.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Alleviation of cadmium uptake in rice (Oryza sativa L.) by iron plaque on the root surface generated by Providencia manganoxydans via Fe(II) oxidation.Arch Microbiol. 2024 Aug 28;206(9):387. doi: 10.1007/s00203-024-04110-4. Arch Microbiol. 2024. PMID: 39196357
-
The diversity and abundance of As(III) oxidizers on root iron plaque is critical for arsenic bioavailability to rice.Sci Rep. 2015 Sep 1;5:13611. doi: 10.1038/srep13611. Sci Rep. 2015. PMID: 26324258 Free PMC article.
-
Rhizosphere iron and manganese-oxidizing bacteria stimulate root iron plaque formation and regulate Cd uptake of rice plants (Oryza sativa L.).J Environ Manage. 2021 Jan 15;278(Pt 2):111533. doi: 10.1016/j.jenvman.2020.111533. Epub 2020 Nov 5. J Environ Manage. 2021. PMID: 33157466
-
Influence of water management on the active root-associated microbiota involved in arsenic, iron, and sulfur cycles in rice paddies.Appl Microbiol Biotechnol. 2017 Sep;101(17):6725-6738. doi: 10.1007/s00253-017-8382-6. Epub 2017 Jun 28. Appl Microbiol Biotechnol. 2017. PMID: 28660288
-
Effects of cadmium and flooding on the formation of iron plaques, the rhizosphere bacterial community structure, and root exudates in Kandelia obovata seedlings.Sci Total Environ. 2022 Dec 10;851(Pt 1):158190. doi: 10.1016/j.scitotenv.2022.158190. Epub 2022 Aug 20. Sci Total Environ. 2022. PMID: 35995174
Cited by
-
Custom-made medium approach for effective enrichment and isolation of chemolithotrophic iron-oxidizing bacteria.FEMS Microbiol Ecol. 2025 May 20;101(6):fiaf051. doi: 10.1093/femsec/fiaf051. FEMS Microbiol Ecol. 2025. PMID: 40328454 Free PMC article.
-
Nitrous oxide is the main product during nitrate reduction by a novel lithoautotrophic iron(II)-oxidizing culture from an organic-rich paddy soil.Appl Environ Microbiol. 2025 Jan 31;91(1):e0126224. doi: 10.1128/aem.01262-24. Epub 2024 Dec 6. Appl Environ Microbiol. 2025. PMID: 39641603 Free PMC article.
-
Microbial magnetite oxidation via MtoAB porin-multiheme cytochrome complex in Sideroxydans lithotrophicus ES-1.Appl Environ Microbiol. 2025 Apr 23;91(4):e0186524. doi: 10.1128/aem.01865-24. Epub 2025 Mar 5. Appl Environ Microbiol. 2025. PMID: 40042276 Free PMC article.
-
Gallionellaceae pangenomic analysis reveals insight into phylogeny, metabolic flexibility, and iron oxidation mechanisms.mSystems. 2023 Dec 21;8(6):e0003823. doi: 10.1128/msystems.00038-23. Epub 2023 Oct 26. mSystems. 2023. PMID: 37882557 Free PMC article.
-
Concentrations and Health Implications of As, Hg, and Cd and Micronutrients in Rice and Emissions of CH4 From Variably Flooded Paddies.Geohealth. 2025 Aug 13;9(8):e2025GH001410. doi: 10.1029/2025GH001410. eCollection 2025 Aug. Geohealth. 2025. PMID: 40810033 Free PMC article.
References
-
- Khan N, Seshadri B, Bolan N, Saint CP, Kirkham MB, Chowdhury S, Yamaguchi N, Lee DY, Li G, Kunhikrishnan A, Qi F, Karunanithi R, Qiu R, Zhu Y-G, Syu CH. 2016. Root iron plaque on wetland plants as a dynamic pool of nutrients and contaminants, p 1–96. In Advances in agronomy. Elsevier.
-
- Ye ZH, Baker AJM, Wong MH, Willis AJ. 1998. Zinc, lead and cadmium accumulation and tolerance in Typha latifolia as affected by iron plaque on the root surface. Aqua Bot 61:55–67. doi:10.1016/S0304-3770(98)00057-6 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

