Characterization of HcaA, a novel autotransporter protein in Helicobacter cinaedi, and its role in host cell adhesion
- PMID: 38009997
- PMCID: PMC10732068
- DOI: 10.1128/msphere.00403-23
Characterization of HcaA, a novel autotransporter protein in Helicobacter cinaedi, and its role in host cell adhesion
Abstract
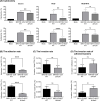
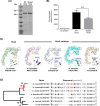
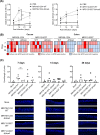
Helicobacter species are classified as gastric or enterohepatic according to their habitat. Among enterohepatic Helicobacter species, which inhabit the intestine, colon, and liver, Helicobacter cinaedi has been most frequently isolated from humans. H. cinaedi often causes bacteremia and cellulitis in immunocompromised hosts. Here, we focused on the H. cinaedi autotransporter protein A (HcaA), a novel virulence factor in H. cinaedi. We discovered that HcaA contributes to cell adhesion via its Arg-Gly-Asp motif. Furthermore, in animal experiments, bacterial colonization was reduced in mice infected with HcaA-knockout strains, supporting the hypothesis that HcaA contributes to H. cinaedi adhesion to host cells. Our study provides a novel mechanism for the establishment of H. cinaedi infections and provides new insights into the role of autotransporter proteins in the establishment of Helicobacter infection.
Keywords: Helicobacter cinaedi; autotransporter protein; cell adhesion; non-Helicobacter pylori Helicobacter species; type V secretion system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Helicobacter cinaedi Hepatic Cyst Infection with Bacteremia.Emerg Infect Dis. 2019 Mar;25(3):603-604. doi: 10.3201/eid2503.180936. Emerg Infect Dis. 2019. PMID: 30789337 Free PMC article.
-
Helicobacter cinaedi is a human-adapted lineage in the Helicobacter cinaedi/canicola/'magdeburgensis' complex.Microb Genom. 2022 May;8(5):mgen000830. doi: 10.1099/mgen.0.000830. Microb Genom. 2022. PMID: 35536747 Free PMC article.
-
Helicobacter cinaedi bacteremia with cellulitis after ABO-incompatible living-donor liver transplantation: Case report.World J Gastroenterol. 2015 Jul 7;21(25):7911-5. doi: 10.3748/wjg.v21.i25.7911. World J Gastroenterol. 2015. PMID: 26167092 Free PMC article.
-
First case of bacteremia caused by Helicobacter cinaedi in a patient with liver cirrhosis: a case report and literature review.Clin J Gastroenterol. 2015 Oct;8(5):306-17. doi: 10.1007/s12328-015-0600-0. Epub 2015 Sep 5. Clin J Gastroenterol. 2015. PMID: 26342291 Review.
-
Infections With Enterohepatic Non-H. pylori Helicobacter Species in X-Linked Agammaglobulinemia: Clinical Cases and Review of the Literature.Front Cell Infect Microbiol. 2022 Feb 4;11:807136. doi: 10.3389/fcimb.2021.807136. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35186782 Free PMC article. Review.
Cited by
-
Helicobacter cinaedi bacterium association with atherosclerosis and other diseases.Front Microbiol. 2024 Apr 8;15:1371717. doi: 10.3389/fmicb.2024.1371717. eCollection 2024. Front Microbiol. 2024. PMID: 38650874 Free PMC article. Review.
References
-
- Matsuo T, Mori N, Mizuno A, Sakurai A, Kawai F, Starkey J, Ohkushi D, Abe K, Yamasaki M, Ito J, Yoshino K, Mikami Y, Uehara Y, Furukawa K. 2020. Infected aortic aneurysm caused by Helicobacter cinaedi: case series and systematic review of the literature. BMC Infect Dis 20:854. doi:10.1186/s12879-020-05582-7 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 16K18928/MEXT | Japan Society for the Promotion of Science (JSPS)
- 19K07074/MEXT | Japan Society for the Promotion of Science (JSPS)
- 22K15465/MEXT | Japan Society for the Promotion of Science (JSPS)
- 2017/GSK | GlaxoSmithKline Japan (GSK)
- JP20fk0108148/Japan Agency for Medical Research and Development (AMED)
LinkOut - more resources
Full Text Sources
Medical

