Thyroid hormone increases fatty acid use in fetal ovine cardiac myocytes
- PMID: 38010207
- PMCID: PMC10680578
- DOI: 10.14814/phy2.15865
Thyroid hormone increases fatty acid use in fetal ovine cardiac myocytes
Abstract
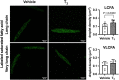
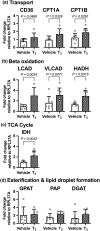
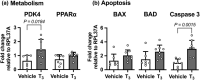
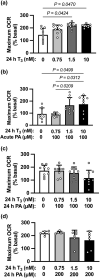
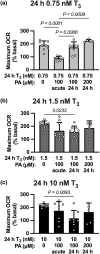
Cardiac metabolic substrate preference shifts at parturition from carbohydrates to fatty acids. We hypothesized that thyroid hormone (T3 ) and palmitic acid (PA) stimulate fetal cardiomyocyte oxidative metabolism capacity. T3 was infused into fetal sheep to a target of 1.5 nM. Dispersed cardiomyocytes were assessed for lipid uptake and droplet formation with BODIPY-labeled fatty acids. Myocardial expression levels were assessed PCR. Cardiomyocytes from naïve fetuses were exposed to T3 and PA, and oxygen consumption was measured with the Seahorse Bioanalyzer. Cardiomyocytes (130-day gestational age) exposed to elevated T3 in utero accumulated 42% more long-chain fatty acid droplets than did cells from vehicle-infused fetuses. In utero T3 increased myocardial mRNA levels of CD36, CPT1A, CPT1B, LCAD, VLCAD, HADH, IDH, PDK4, and caspase 9. In vitro exposure to T3 increased maximal oxygen consumption rate in cultured cardiomyocytes in the absence of fatty acids, and when PA was provided as an acute (30 min) supply of cellular energy. Longer-term exposure (24 and 48 h) to PA abrogated increased oxygen consumption rates stimulated by elevated levels of T3 in cultured cardiomyocytes. T3 contributes to metabolic maturation of fetal cardiomyocytes. Prolonged exposure of fetal cardiomyocytes to PA, however, may impair oxidative capacity.
Keywords: fetus; heart; heart metabolism; lipid metabolism.
© 2023 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
None of the authors have any competing interest to declare.
Figures
Similar articles
-
Lipid exposure leads to metabolic dysfunction in fetal sheep cardiomyocytes.Physiol Rep. 2025 May;13(10):e70386. doi: 10.14814/phy2.70386. Physiol Rep. 2025. PMID: 40420618 Free PMC article.
-
Maturation of lipid metabolism in the fetal and newborn sheep heart.Am J Physiol Regul Integr Comp Physiol. 2023 Dec 1;325(6):R809-R819. doi: 10.1152/ajpregu.00122.2023. Epub 2023 Oct 23. Am J Physiol Regul Integr Comp Physiol. 2023. PMID: 37867472 Free PMC article.
-
Thyroid hormone receptor function in maturing ovine cardiomyocytes.J Physiol. 2019 Apr;597(8):2163-2176. doi: 10.1113/JP276874. Epub 2019 Mar 20. J Physiol. 2019. PMID: 30770568 Free PMC article.
-
Thyroid hormone signaling and consequences for cardiac development.J Endocrinol. 2019 Jul 1;242(1):T145-T160. doi: 10.1530/JOE-18-0704. J Endocrinol. 2019. PMID: 31117055 Free PMC article. Review.
-
Human embryonic stem cell-derived cardiomyocytes as an in vitro model to study cardiac insulin resistance.Biochim Biophys Acta Mol Basis Dis. 2018 May;1864(5 Pt B):1960-1967. doi: 10.1016/j.bbadis.2017.12.025. Epub 2017 Dec 20. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29277329 Review.
Cited by
-
Sensitivity and activation of endoplasmic reticulum stress response and apoptosis in the perinatal sheep heart.Am J Physiol Heart Circ Physiol. 2024 Jul 1;327(1):H1-H11. doi: 10.1152/ajpheart.00043.2024. Epub 2024 May 3. Am J Physiol Heart Circ Physiol. 2024. PMID: 38700493 Free PMC article.
-
Lipid exposure leads to metabolic dysfunction in fetal sheep cardiomyocytes.Physiol Rep. 2025 May;13(10):e70386. doi: 10.14814/phy2.70386. Physiol Rep. 2025. PMID: 40420618 Free PMC article.
-
Maternal high fat-high energy diet alters metabolic factors in the non-human primate fetal heart.J Physiol. 2024 Sep;602(17):4251-4269. doi: 10.1113/JP286861. Epub 2024 Aug 1. J Physiol. 2024. PMID: 39087821 Free PMC article.
-
Gestational Diabetes-like Fuels Impair Mitochondrial Function and Long-Chain Fatty Acid Uptake in Human Trophoblasts.Int J Mol Sci. 2024 Oct 27;25(21):11534. doi: 10.3390/ijms252111534. Int J Mol Sci. 2024. PMID: 39519087 Free PMC article.
References
-
- Bartelds, B. , Knoester, H. , Smid, G. B. , Takens, J. , Visser, G. H. , Penninga, L. , van der Leij, F. R. , Beaufort‐Krol, G. C. , Zijlstra, W. G. , Heymans, H. S. , & Kuipers, J. R. (2000). Perinatal changes in myocardial metabolism in lambs. Circulation, 102, 926–931. - PubMed
-
- Chattergoon, N. N. , Giraud, G. D. , & Thornburg, K. L. (2007). Thyroid hormone inhibits proliferation of fetal cardiac myocytes in vitro. The Journal of Endocrinology, 192, R1–R8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

