FDA Approval Summary: Ivosidenib in Combination with Azacitidine for Treatment of Patients with Newly Diagnosed Acute Myeloid Leukemia with an IDH1 Mutation
- PMID: 38010220
- PMCID: PMC10984783
- DOI: 10.1158/1078-0432.CCR-23-2234
FDA Approval Summary: Ivosidenib in Combination with Azacitidine for Treatment of Patients with Newly Diagnosed Acute Myeloid Leukemia with an IDH1 Mutation
Abstract
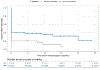
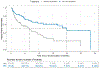
On May 25, 2022, FDA approved a supplemental application for ivosidenib (Tibsovo; Servier) extending the indication in patients with newly diagnosed IDH1-mutated acute myeloid leukemia (AML) in older adults or those with comorbidities to include the combination with azacitidine. The efficacy of ivosidenib in combination with azacitidine was evaluated in Study AG120-C-009, a phase 3, multicenter, double-blind, randomized (1:1), controlled study of ivosidenib or matched placebo in combination with azacitidine in adults with previously untreated AML with an IDH1 mutation who were 75 years or older or had comorbidities that precluded use of intensive induction chemotherapy. Efficacy was established on the basis of improved event-free survival and overall survival on the ivosidenib + azacitidine arm [HR, 0.35; 95% confidence interval (CI), 0.17-0.72; P = 0.0038, and HR, 0.44; 95% CI, 0.27-0.73; P = 0.0010], respectively. Furthermore, the rate and duration of complete remission (CR) were improved with ivosidenib versus placebo [CR 47% versus 15%, two-sided P < 0.0001; median duration of CR not estimable (NE; 95% CI, 13.0-NE) months versus 11.2 (95% CI, 3.2-NE) months. The safety profile of ivosidenib in combination with azacitidine was consistent with that of ivosidenib monotherapy, with important adverse reactions including differentiation syndrome (15%) and QT interval prolongation (20%).
Trial registration: ClinicalTrials.gov NCT03173248 NCT02677922.
©2023 American Association for Cancer Research.
Conflict of interest statement
Figures
References
-
- SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2018 Sub (1975–2016) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission.
-
- DiNardo CD, Wei AH; How I treat acute myeloid leukemia in the era of new drugs. Blood 2020; 135 (2): 85–96. - PubMed
-
- Amadori S, Suciu S, Selleslag D, Aversa F, Gaidano G, Musso M, et al. Gemtuzumab Ozogamicin Versus Best Supportive Care in Older Patients With Newly Diagnosed Acute Myeloid Leukemia Unsuitable for Intensive Chemotherapy: Results of the Randomized Phase III EORTC-GIMEMA AML-19 Trial. J Clin Oncol. 2016. Mar 20;34(9):972–9. - PubMed
-
- Ferrara F, Barosi G, Venditti A, Angelucci E, Gobbi M, Pane F, Tosi P, Zinzani P, Tura S. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: a project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia. 2013. Apr;27(5):997–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

