High-dose chemotherapy with autologous stem-cell transplantation for relapsed metastatic germ cell tumors The Alberta experience
- PMID: 38010229
- PMCID: PMC10954282
- DOI: 10.5489/cuaj.8493
High-dose chemotherapy with autologous stem-cell transplantation for relapsed metastatic germ cell tumors The Alberta experience
Abstract
Introduction: High-dose chemotherapy with autologous stem-cell transplantation (HDC-ASCT) is standard therapy for metastatic germ cell tumors (mGCTs) in patients whose disease progresses during or after conventional chemotherapy. We conducted a retrospective review of HDC-ASCT in relapsed mGCT patients in the province of Alberta, Canada, over the past two decades.
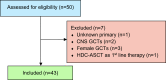
Methods: Patients with mGCTs who received HDC-ASCT at two provincial cancer referral centers from 2000-2018 were identified from institutional databases. Baseline clinical and treatment characteristics were collected, as well as overall survival (OS ) and disease-free survival (DFS). Relevant prognostic variables were analyzed.
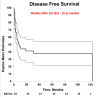
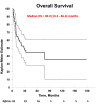
Results: Forty-three patients were identified. The median age was 28 years (range 19-56). A majority (95%) had non-seminoma histology and testis/retroperitoneal primary (84%). Twenty patients (47%) had poor-risk disease, as per The International Germ Cell Consensus Classification (IGCCC), at start of first-line chemotherapy. HDC-ASCT was used as second-line therapy in 65% of patients, and 58% of ASCT patients received tandem transplants. Median followup after ASCT was 22 months (range 2-181). At last followup, 42% of patients were alive without disease, including 3/7 (43%) of patients with primary mediastinal disease. Two-year and five-year DFS/OS ratios were 44%/65% and 38%/45%, respectively. Median OS and DFS for all patients were 30.0 months (13.3-46.6) and 8.0 months (0.9-15.1), respectively.
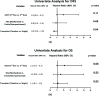
Conclusions: We found that HDC-ASCT is an effective salvage therapy in mGCT, consistent with existing literature. Patients appeared to benefit regardless of primary site. Although limited by small sample size, we found a numerical difference in DFS and OS between second- and third-line HDC-ASCT and single vs. tandem ASCT.
Conflict of interest statement
COMPETING INTERESTS: Dr. Zhang has participated in advisory board for Novartis AAA, and Pfizer. Dr. Alimohamed has had advisory/consultancy roles with AstraZeneca, Bayer, BMS, EMD Serono, Gilead, Merck, Pfizer, and Seagen. Dr. Basappa has been an advisory board member for AstraZeneca, Bayer, BMS, Eisai, EMD Serono, Ipsen, Janssen, Merck, Pfizer, Roche, and Seagen; and has received travel support from Eisai and Janssen. Dr. Heng has been an advisory board member for Bayer, BMS, Eisai, Exilexis, Ipsen, KGA, Merck, Novartis, and Pfizer. Dr. North has received honoraria from AAA, Astellas, BMS, EMD Serono, Janssen, Merck, and Roche. Dr. Kolinsky has received honoraria from Astellas, AstraZeneca, Bayer, BMS, Eisai, EMD Serono, Ipsen, Janssen, and Merck; and has participated in clinical trials supported by Astellas, AstraZeneca, Bayer, BMS, Eisai, EMD Serono, Ipsen, Janssen, Merck, and Seattle Genetics. The remaining authors do not report any competing personal or financial interests related to this work.
Figures
References
-
- Average years of life lost per person dying of cancer, all races, both sexes, 2004 SEER DATABASE 1975–2004. [Accessed Nov 20, 2023]. Published NIH 2004. Available at: http://seer.cancer.gov/csr/1975_2004/results_figure/sect_01_zfig.19.pdf.
LinkOut - more resources
Full Text Sources
Miscellaneous
