Patient-Derived Microphysiological Systems for Precision Medicine
- PMID: 38010253
- PMCID: PMC11469251
- DOI: 10.1002/adhm.202303161
Patient-Derived Microphysiological Systems for Precision Medicine
Abstract
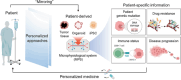
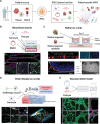
Patient-derived microphysiological systems (P-MPS) have emerged as powerful tools in precision medicine that provide valuable insight into individual patient characteristics. This review discusses the development of P-MPS as an integration of patient-derived samples, including patient-derived cells, organoids, and induced pluripotent stem cells, into well-defined MPSs. Emphasizing the necessity of P-MPS development, its significance as a nonclinical assessment approach that bridges the gap between traditional in vitro models and clinical outcomes is highlighted. Additionally, guidance is provided for engineering approaches to develop microfluidic devices and high-content analysis for P-MPSs, enabling high biological relevance and high-throughput experimentation. The practical implications of the P-MPS are further examined by exploring the clinically relevant outcomes obtained from various types of patient-derived samples. The construction and analysis of these diverse samples within the P-MPS have resulted in physiologically relevant data, paving the way for the development of personalized treatment strategies. This study describes the significance of the P-MPS in precision medicine, as well as its unique capacity to offer valuable insights into individual patient characteristics.
Keywords: microphysiological system; patient-derived; physiologically-relevant; precision medicine.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Organoids-On-a-Chip for Personalized Precision Medicine.Adv Healthc Mater. 2024 Dec;13(30):e2401843. doi: 10.1002/adhm.202401843. Epub 2024 Oct 13. Adv Healthc Mater. 2024. PMID: 39397335 Review.
-
[The potential of neural microphysiological systems (MPS)].Nihon Yakurigaku Zasshi. 2025;160(2):92-96. doi: 10.1254/fpj.24098. Nihon Yakurigaku Zasshi. 2025. PMID: 40024712 Japanese.
-
A path forward advancing microphysiological systems.ALTEX. 2025;42(2):183-203. doi: 10.14573/altex.2504091. ALTEX. 2025. PMID: 40231859
-
Fitting tissue chips and microphysiological systems into the grand scheme of medicine, biology, pharmacology, and toxicology.Exp Biol Med (Maywood). 2017 Oct;242(16):1559-1572. doi: 10.1177/1535370217732765. Exp Biol Med (Maywood). 2017. PMID: 29065799 Free PMC article.
-
Advancements in Microphysiological systems: Exploring organoids and organ-on-a-chip technologies in drug development -focus on pharmacokinetics related organs.Drug Metab Pharmacokinet. 2025 Feb;60:101046. doi: 10.1016/j.dmpk.2024.101046. Epub 2024 Dec 17. Drug Metab Pharmacokinet. 2025. PMID: 39847980 Review.
Cited by
-
Bio-orthogonal tuning of matrix properties during 3D cell culture to induce morphological and phenotypic changes.Nat Protoc. 2025 Mar;20(3):727-778. doi: 10.1038/s41596-024-01066-z. Epub 2024 Nov 5. Nat Protoc. 2025. PMID: 39501109 Review.
-
Dynamic microphysiological system chip platform for high-throughput, customizable, and multi-dimensional drug screening.Bioact Mater. 2024 May 17;39:59-73. doi: 10.1016/j.bioactmat.2024.05.019. eCollection 2024 Sep. Bioact Mater. 2024. PMID: 38800720 Free PMC article.
-
Precision Treatment of Metachronous Multiple Primary Malignancies Based on Constructing Patient Tumor-Derived Organoids.Biomedicines. 2024 Nov 27;12(12):2708. doi: 10.3390/biomedicines12122708. Biomedicines. 2024. PMID: 39767614 Free PMC article.
-
Integrated Patient Digital and Biomimetic Twins for Precision Medicine: A Perspective.Semin Liver Dis. 2025 Jul 23:10.1055/a-2649-1560. doi: 10.1055/a-2649-1560. Online ahead of print. Semin Liver Dis. 2025. PMID: 40614771 Free PMC article.
-
Liver-Skin Microphysiological System for Evaluating Topical Drug Delivery-Induced Liver Injury.ACS Omega. 2025 Jun 2;10(23):24284-24295. doi: 10.1021/acsomega.5c00222. eCollection 2025 Jun 17. ACS Omega. 2025. PMID: 40547626 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

