Bicyclopentylation of Alcohols with Thianthrenium Reagents
- PMID: 38010346
- PMCID: PMC10704608
- DOI: 10.1021/jacs.3c10024
Bicyclopentylation of Alcohols with Thianthrenium Reagents
Erratum in
-
Addition to "Bicyclopentylation of Alcohols with Thianthrenium Reagents".J Am Chem Soc. 2024 Jan 24;146(3):2288. doi: 10.1021/jacs.4c00054. Epub 2024 Jan 11. J Am Chem Soc. 2024. PMID: 38212883 Free PMC article. No abstract available.
Abstract
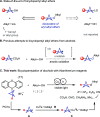
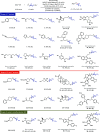
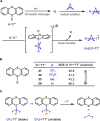
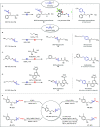
Herein we present the first method for the synthesis of bicyclo[1.1.1]pentyl (BCP) alkyl ethers from alcohols. The reaction uses BCP-thianthrenium reagents and is catalyzed by a dual copper/photoredox catalyst system. Unlike known alkylations of tertiary alcohols via carbocation intermediates, our Cu-mediated radical process circumvents the labile BCP carbocations. The approach demonstrates a broad tolerance for functional groups when applied to primary, secondary, and even tertiary alcohols. In addition, we highlight the utility of this method in late-stage functionalizations of both natural products and pharmaceuticals as well as in the rapid construction of BCP analogs of known pharmaceuticals that would otherwise be difficult to access.
Conflict of interest statement
The authors declare the following competing financial interest(s): T.R. and Z.B. may benefit from thianthrene compound-based sales.
Figures

References
-
- Pellicciari R.; Raimondo M.; Marinozzi M.; Natalini B.; Costantino G.; Thomsen C. (S)-(+)-2-(3′-Carboxybicyclo[1.1.1]pentyl)-glycine, a Structurally New Group I Metabotropic Glutamate Receptor Antagonist. J. Med. Chem. 1996, 39, 2874–2876. 10.1021/jm960254o. - DOI - PubMed
- Filosa R.; Carmela Fulco M.; Marinozzi M.; Giacchè N.; Macchiarulo A.; Peduto A.; Massa A.; de Caprariis P.; Thomsen C.; Christoffersen C. T.; Pellicciari R. Design, synthesis and biological evaluation of novel bicyclo[1.1.1]pentane-based ω-acidic amino acids as glutamate receptors ligands. Bioorg. Med. Chem. 2009, 17, 242–250. 10.1016/j.bmc.2008.11.015. - DOI - PubMed
-
- Stepan A. F.; Subramanyam C.; Efremov I. V.; Dutra J. K.; O’Sullivan T. J.; DiRico K. J.; McDonald W. S.; Won A.; Dorff P. H.; Nolan C. E.; Becker S. L.; Pustilnik L. R.; Riddell D. R.; Kauffman G. W.; Kormos B. L.; Zhang L.; Lu Y.; Capetta S. H.; Green M. E.; Karki K.; Sibley E.; Atchison K. P.; Hallgren A. J.; Oborski C. E.; Robshaw A. E.; Sneed B.; O’Donnell C. J. Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active gamma-secretase inhibitor. J. Med. Chem. 2012, 55, 3414–3424. 10.1021/jm300094u. - DOI - PubMed
- Auberson Y. P.; Brocklehurst C.; Furegati M.; Fessard T. C.; Koch G.; Decker A.; La Vecchia L.; Briard E. Improving Nonspecific Binding and Solubility: Bicycloalkyl Groups and Cubanes as para-Phenyl Bioisosteres. ChemMedChem 2017, 12, 590–598. 10.1002/cmdc.201700082. - DOI - PubMed
- Goh Y. L.; Cui Y. T.; Pendharkar V.; Adsool V. A. Toward Resolving the Resveratrol Conundrum: Synthesis and in Vivo Pharmacokinetic Evaluation of BCP-Resveratrol. ACS Med. Chem. Lett. 2017, 8, 516–520. 10.1021/acsmedchemlett.7b00018. - DOI - PMC - PubMed
- Measom N. D.; Down K. D.; Hirst D. J.; Jamieson C.; Manas E. S.; Patel V. K.; Somers D. O. Investigation of a Bicyclo[1.1.1]pentane as a Phenyl Replacement within a LpPLA2 Inhibitor. ACS Med. Chem. Lett. 2017, 8, 43–48. 10.1021/acsmedchemlett.6b00281. - DOI - PMC - PubMed
-
- Gianatassio R.; Lopchuk J. M.; Wang J.; Pan C.-M.; Malins L. R.; Prieto L.; Brandt T. A.; Collins M. R.; Gallego G. M.; Sach N. W.; Spangler J. E.; Zhu H.; Zhu J.; Baran P. S. Strain-release amination. Science 2016, 351, 241–246. 10.1126/science.aad6252. - DOI - PMC - PubMed
- Kanazawa J.; Maeda K.; Uchiyama M. Radical Multicomponent Carboamination of [1.1.1]Propellane. J. Am. Chem. Soc. 2017, 139, 17791–17794. 10.1021/jacs.7b11865. - DOI - PubMed
- Hughes J. M. E.; Scarlata D. A.; Chen A. C.; Burch J. D.; Gleason J. L. Aminoalkylation of [1.1.1]Propellane Enables Direct Access to High-Value 3-Alkylbicyclo[1.1.1]pentan-1-amines. Org. Lett. 2019, 21, 6800–6804. 10.1021/acs.orglett.9b02426. - DOI - PubMed
- Zhang X.; Smith R. T.; Le C.; McCarver S. J.; Shireman B. T.; Carruthers N. I.; MacMillan D. W. C. Copper-mediated synthesis of drug-like bicyclopentanes. Nature 2020, 580, 220–226. 10.1038/s41586-020-2060-z. - DOI - PMC - PubMed
- Pickford H. D.; Nugent J.; Owen B.; Mousseau J. J.; Smith R. C.; Anderson E. A. Twofold Radical-Based Synthesis of N,C-Difunctionalized Bicyclo[1.1.1]pentanes. J. Am. Chem. Soc. 2021, 143, 9729–9736. 10.1021/jacs.1c04180. - DOI - PubMed
- Shin S.; Lee S.; Choi W.; Kim N.; Hong S. Visible-Light-Induced 1,3-Aminopyridylation of [1.1.1]Propellane with N-Aminopyridinium Salts. Angew. Chem., Int. Ed. 2021, 60, 7873–7879. 10.1002/anie.202016156. - DOI - PubMed
- Livesley S.; Sterling A. J.; Robertson C. M.; Goundry W. R. F.; Morris J. A.; Duarte F.; Aissa C. Electrophilic Activation of [1.1.1]Propellane for the Synthesis of Nitrogen-Substituted Bicyclo[1.1.1]pentanes. Angew. Chem., Int. Ed. 2022, 61, e202111291 10.1002/anie.202111291. - DOI - PMC - PubMed
- Alvarez E. M.; Bai Z.; Pandit S.; Frank N.; Torkowski L.; Ritter T. O-, N- and C-bicyclopentylation using thianthrenium reagents. Nat. Synth. 2023, 2, 548–556. 10.1038/s44160-023-00277-8. - DOI
LinkOut - more resources
Full Text Sources

