Pharmacodynamic effects of the PARP inhibitor talazoparib (MDV3800, BMN 673) in patients with BRCA-mutated advanced solid tumors
- PMID: 38010394
- PMCID: PMC10902014
- DOI: 10.1007/s00280-023-04600-0
Pharmacodynamic effects of the PARP inhibitor talazoparib (MDV3800, BMN 673) in patients with BRCA-mutated advanced solid tumors
Abstract
Purpose: Talazoparib is an inhibitor of the poly (ADP-ribose) polymerase (PARP) family of enzymes and is FDA-approved for patients with (suspected) deleterious germline BRCA1/2-mutated, HER2‑negative, locally advanced or metastatic breast cancer. Because knowledge of the pharmacodynamic (PD) effects of talazoparib in patients has been limited to studies of PARP enzymatic activity (PARylation) in peripheral blood mononuclear cells, we developed a study to assess tumoral PD response to talazoparib treatment (NCT01989546).
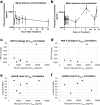
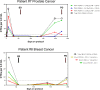
Methods: We administered single-agent talazoparib (1 mg/day) orally in 28-day cycles to adult patients with advanced solid tumors harboring (suspected) deleterious BRCA1 or BRCA2 mutations. The primary objective was to examine the PD effects of talazoparib; the secondary objective was to determine overall response rate (ORR). Tumor biopsies were mandatory at baseline and post-treatment on day 8 (optional at disease progression). Biopsies were analyzed for PARylation, DNA damage response (γH2AX), and epithelial‒mesenchymal transition.
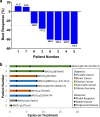
Results: Nine patients enrolled in this trial. Four of six patients (67%) evaluable for the primary PD endpoint exhibited a nuclear γH2AX response on day 8 of treatment, and five of six (83%) also exhibited strong suppression of PARylation. A transition towards a more mesenchymal phenotype was seen in 4 of 6 carcinoma patients, but this biological change did not affect γH2AX or PAR responses. The ORR was 55% with the five partial responses lasting a median of six cycles.
Conclusion: Intra-tumoral DNA damage response and inhibition of PARP enzymatic activity were confirmed in patients with advanced solid tumors harboring BRCA1/2 mutations after 8 days of talazoparib treatment.
Keywords: Clinical trial; DNA damage repair; Homologous recombination repair; Pharmacology; Poly-ADP-ribosylation; Targeted agent.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures


References
-
- Schreiber V, Ame JC, Dolle P, Schultz I, Rinaldi B, Fraulob V, Menissier-de Murcia J, de Murcia G. Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J Biol Chem. 2002;277(25):23028–23036. doi: 10.1074/jbc.M202390200. - DOI - PubMed
-
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. doi: 10.1038/nature03445. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

