Rituximab in combination with cyclosporine and steroid pulse therapy for childhood-onset multidrug-resistant nephrotic syndrome: a multicenter single-arm clinical trial (JSKDC11 trial)
- PMID: 38010466
- PMCID: PMC10955017
- DOI: 10.1007/s10157-023-02431-0
Rituximab in combination with cyclosporine and steroid pulse therapy for childhood-onset multidrug-resistant nephrotic syndrome: a multicenter single-arm clinical trial (JSKDC11 trial)
Abstract
Background: Only 80% of children with idiopathic nephrotic syndrome respond well to glucocorticoid therapy. Multidrug-resistant nephrotic syndrome (MRNS) is associated with a poor kidney prognosis. Several retrospective studies have identified rituximab as an effective treatment for MRNS; however, prospective studies are required to assess its efficacy and safety.
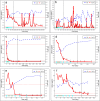
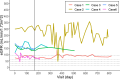
Methods: We conducted a multicenter, non-blinded, single-arm trial to investigate the efficacy and safety of rituximab in patients with childhood-onset MRNS who were resistant to cyclosporine and more than three courses of steroid pulse therapy. The enrolled patients received four 375 mg/m2 doses of rituximab in combination with baseline cyclosporine and steroid pulse therapy. The primary endpoint was a > 50% reduction in the urinary protein/creatinine ratio from baseline on day 169. Complete and partial remissions were also evaluated.
Results: Six patients with childhood-onset MRNS were enrolled. All patients were negative for pathogenic variants of podocyte-related genes. On day 169, five patients (83.3%) showed a > 50% reduction in the urinary protein/creatinine ratio, two patients showed partial remission, and two patients showed complete remission. No deaths occurred and severe adverse events occurred in two patients (infection in one patient and acute kidney injury in one patient). Three patients needed treatment for moderate-to-severe infection.
Conclusions: The study treatment effectively reduced the urinary protein/creatinine ratio in patients with childhood-onset MRNS. The adverse events in this study were within the expected range; however, attention should be paid to the occurrence of infections.
Keywords: Cyclosporine; MRNS; Methylprednisolone; Rituximab; SRNS.
© 2023. The Author(s).
Conflict of interest statement
Kandai Nozu received lecture fees from Chugai Pharmaceutical Co., Ltd. Mayumi Sako received consulting fees from Zenyaku Kogyo Co., Ltd. Koichi Kamei received grants from Chugai Pharmaceutical Co., Ltd. and Pfizer Inc., as well as lecture and/or consulting fees from Chugai Pharmaceutical Co., Ltd., Zenyaku Kogyo Co., Ltd., and Novartis Pharma K.K. Riku Hamada received lecture fees from Chugai Pharmaceutical Co., Ltd. and Novartis Pharma K.K. Yuko Shima received lecture fees from Novartis Pharma K.K. Yasufumi Ohtsuka received lecture fees from Chugai Pharmaceutical Co. Kenji Ishikura received grants from Novartis Pharma K.K., Zenyaku Kogyo Co., Ltd., and Chugai Pharmaceutical Co., as well as lecture fees from Chugai Pharmaceutical Co., Zenyaku Kogyo Co., and Novartis Pharma K.K. Koichi Nakanishi received lecture fees from Chugai Pharmaceutical Co., Ltd. and Novartis Pharma K.K. Kazumoto Iijima received a grant from Zenyaku Kogyo Co., Ltd., as well as lecture and/or consulting fees from Chugai Pharmaceutical Co., Ltd., Zenyaku Kogyo Co., Ltd., and Novartis Pharma K.K.
Figures

References
-
- Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, et al. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100(4):753–79. 10.1016/j.kint.2021.05.015. - PubMed
-
- Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003;362(9384):629–39. 10.1016/S0140-6736(03)14184-0. - PubMed
-
- Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol. 1997;8(5):769–76. - PubMed
-
- Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft fur Padiatrische Nephrologie. Lancet. 1988;1(8582):380–3 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

