mRNA vaccine development during the COVID-19 pandemic: a retrospective review from the perspective of the Swiss affiliate of a global biopharmaceutical company
- PMID: 38012751
- PMCID: PMC10683226
- DOI: 10.1186/s40545-023-00652-y
mRNA vaccine development during the COVID-19 pandemic: a retrospective review from the perspective of the Swiss affiliate of a global biopharmaceutical company
Erratum in
-
Correction: mRNA vaccine development during the COVID-19 pandemic: a retrospective review from the perspective of the Swiss affiliate of a global biopharmaceutical company.J Pharm Policy Pract. 2023 Dec 11;16(1):167. doi: 10.1186/s40545-023-00677-3. J Pharm Policy Pract. 2023. PMID: 38082358 Free PMC article. No abstract available.
Abstract
The coronavirus disease 2019 (COVID-19) pandemic has been the defining public health emergency of our time. In Switzerland, messenger RNA (mRNA) vaccines were and still are widely utilized as a critical component of the Federal Office of Public Health (FOPH)'s preventative mitigation strategy. The development, conditional approval and worldwide roll-out of mRNA vaccines against COVID-19 proceeded at an unprecedented pace and presented myriad challenges for manufacturers. In this review, we discuss, from the perspective of the Swiss affiliate of a global biopharmaceutical company, the clinical, regulatory, pharmacovigilance and logistical considerations of making a mRNA COVID-19 vaccine available to the Swiss population during a pandemic as rapidly as possible while ensuring strict adherence to safety and quality standards.
Trial registration: ClinicalTrials.gov NCT04368728.
Keywords: Clinical development; Coronavirus disease 2019; Messenger ribonucleic acid; Pandemic; Public health; Regulatory; Safety; Supply; Switzerland; Vaccines.
© 2023. Pfizer Inc.
Conflict of interest statement
All authors are employees and shareholders of Pfizer. The conception of and decision to write the article was the authors’ alone. Quality control was conducted by another Pfizer AG employee.
Figures


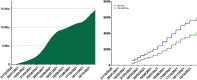
References
-
- Swiss Federal Office of Public Health. Status report, Switzerland and Liechtenstein. COVID-19 Switzerland: Information on the current situation. 2022. https://www.covid19.admin.ch/en/overview. Accessed 15 Nov 2023.
-
- Warren GW, Lofstedt R, Wardman JK. COVID-19: the winter lockdown strategy in five European nations. J Risk Res. 2021;24(3–4):267–293. doi: 10.1080/13669877.2021.1891802. - DOI
-
- Allam Z. The first 50 days of COVID-19: a detailed chronological timeline and extensive review of literature documenting the pandemic. Surv Covid-19 Pandemic its Implic. 2020:1–7.
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical
