Wait Time Advantage for Transplant Candidates With HIV Who Accept Kidneys From Donors With HIV Under the HOPE Act
- PMID: 38012862
- PMCID: PMC11037099
- DOI: 10.1097/TP.0000000000004857
Wait Time Advantage for Transplant Candidates With HIV Who Accept Kidneys From Donors With HIV Under the HOPE Act
Abstract
Background: Kidney transplant (KT) candidates with HIV face higher mortality on the waitlist compared with candidates without HIV. Because the HIV Organ Policy Equity (HOPE) Act has expanded the donor pool to allow donors with HIV (D + ), it is crucial to understand whether this has impacted transplant rates for this population.
Methods: Using a linkage between the HOPE in Action trial (NCT03500315) and Scientific Registry of Transplant Recipients, we identified 324 candidates listed for D + kidneys (HOPE) compared with 46 025 candidates not listed for D + kidneys (non-HOPE) at the same centers between April 26, 2018, and May 24, 2022. We characterized KT rate, KT type (D + , false-positive [FP; donor with false-positive HIV testing], D - [donor without HIV], living donor [LD]) and quantified the association between HOPE enrollment and KT rate using multivariable Cox regression with center-level clustering; HOPE was a time-varying exposure.
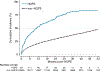
Results: HOPE candidates were more likely male individuals (79% versus 62%), Black (73% versus 35%), and publicly insured (71% versus 52%; P < 0.001). Within 4.5 y, 70% of HOPE candidates received a KT (41% D + , 34% D - , 20% FP, 4% LD) versus 43% of non-HOPE candidates (74% D - , 26% LD). Conversely, 22% of HOPE candidates versus 39% of non-HOPE candidates died or were removed from the waitlist. Median KT wait time was 10.3 mo for HOPE versus 60.8 mo for non-HOPE candidates ( P < 0.001). After adjustment, HOPE candidates had a 3.30-fold higher KT rate (adjusted hazard ratio = 3.30, 95% confidence interval, 2.14-5.10; P < 0.001).
Conclusions: Listing for D + kidneys within HOPE trials was associated with a higher KT rate and shorter wait time, supporting the expansion of this practice for candidates with HIV.
Copyright © 2023 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
D.L.S. receives honoraria for AstraZeneca, CareDx, Houston Methodist, Northwell Health, Optum Health Education, Sanofi, and WebMd, as well as consulting fees from AstraZeneca, CareDx, Moderna Therapeutics, Novavax, Regeneron, and Springer Publishing. C.M.D. received honoraria from Gilead Sciences for serving on a grant review committee. V.S. received research grant support from Eli Lilly and Company and consulting fees from DiaSorin SpA. M.P. received grant support from Merck, Hologic, Moderna, Shire/Takeda, and Pfizer, as well as provided consultation to/serves on the advisory board for Rebiotix, Takeda, and Union Therapeutics. C.B.S. has received grants paid to her institution from GlaxoSmithKline, ViiV, Abbott, Merck, Gilead, Chimerix, Shire/Takeda, Schering-Plough, Ablynx, Janssen, Ansun Biopharma, and Karyopharm Therapeutics. G.H. is a recipient of research grants from Allovir, Karius, and AstraZeneca. G.H. serves on the scientific advisory boards of Karius and AstraZeneca and has received honoraria from MDOutlook and MAD-ID/ID Connect. J.H. is a recipient of grants paid to his institution from Pfizer, Janssen, and Scynexis. The other authors declare no conflicts of interest.
Figures

References
-
- Abraham AG, Althoff KN, Jing Y, et al.; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of the International Epidemiologic Databases to Evaluate AIDS (IeDEA). End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis. 2015;60:941–949. - PMC - PubMed
-
- Bickel M, Marben W, Betz C, et al. End-stage renal disease and dialysis in HIV-positive patients: observations from a long-term cohort study with a follow-up of 22 years. HIV Med. 2013;14:127–135. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

