Effectiveness of low-intensity atorvastatin 5 mg and ezetimibe 10 mg combination therapy compared with moderate-intensity atorvastatin 10 mg monotherapy: A randomized, double-blinded, multi-center, phase III study
- PMID: 38013289
- PMCID: PMC10681377
- DOI: 10.1097/MD.0000000000036122
Effectiveness of low-intensity atorvastatin 5 mg and ezetimibe 10 mg combination therapy compared with moderate-intensity atorvastatin 10 mg monotherapy: A randomized, double-blinded, multi-center, phase III study
Abstract
Background: We compared the efficacy and safety of low-intensity atorvastatin and ezetimibe combination therapy with moderate-intensity atorvastatin monotherapy in patients requiring cholesterol-lowering therapy.
Methods: At 19 centers in Korea, 290 patients were randomized to 4 groups: atorvastatin 5 mg and ezetimibe 10 mg (A5E), ezetimibe 10 mg (E), atorvastatin 5 mg (A5), and atorvastatin 10 mg (A10). Clinical and laboratory examinations were performed at baseline, and at 4-week and 8-week follow-ups. The primary endpoint was percentage change from baseline in low-density lipoprotein (LDL) cholesterol levels at the 8-week follow-up. Secondary endpoints included percentage changes from baseline in additional lipid parameters.
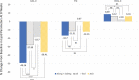
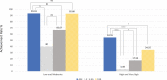
Results: Baseline characteristics were similar among the study groups. At the 8-week follow-up, percentage changes in LDL cholesterol levels were significantly greater in the A5E group (49.2%) than in the E (18.7%), A5 (27.9%), and A10 (36.4%) groups. Similar findings were observed regarding the percentage changes in total cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B levels. Triglyceride levels were also significantly decreased in the A5E group than in the E group, whereas high-density lipoprotein levels substantially increased in the A5E group than in the E group. In patients with low- and intermediate-cardiovascular risk, 93.3% achieved the target LDL cholesterol levels in the A5E group, 40.0% in the E group, 66.7% in the A5 group, and 92.9% in the A10 group. In addition, 31.4% of patients in the A5E group, 8.1% in E, 9.7% in A5, and 7.3% in the A10 group reached the target levels of both LDL cholesterol < 70 mg/dL and reduction of LDL ≥ 50% from baseline.
Conclusions: The addition of ezetimibe to low-intensity atorvastatin had a greater effect on lowering LDL cholesterol than moderate-intensity atorvastatin alone, offering an effective treatment option for cholesterol management, especially in patients with low and intermediate risks.
Copyright © 2023 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88. - PubMed
-
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:3168–209. - PubMed
-
- Nguyen KA, Li L, Lu D, et al. A comprehensive review and meta-analysis of risk factors for statin-induced myopathy. Eur J Clin Pharmacol. 2018;74:1099–109. - PubMed
-
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

