High-performance polyimine vitrimers from an aromatic bio-based scaffold
- PMID: 38013907
- PMCID: PMC10540462
- DOI: 10.1039/d3lp00019b
High-performance polyimine vitrimers from an aromatic bio-based scaffold
Abstract
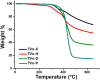
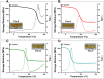
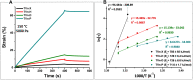
Bio-based vitrimers represent a promising class of thermosetting polymer materials, pairing the recyclability of dynamic covalent networks with the renewability of non-fossil fuel feedstocks. Vanillin, a low-cost lignin derivative, enables facile construction of polyimine networks marked by rapid exchange and sensitivity to acid-catalyzed hydrolysis. Furthermore, the aromatic structure makes it a promising candidate for the design of highly aromatic networks capable of high-performance thermal and dimensional stability. Such properties are paramount in polymeric thermal protection systems. Here, we report on the fabrication of polyimine networks with particularly high aromatic content from a novel trifunctional vanillin monomer prepared from the nucleophilic aromatic substitution of perfluoropyridine (PFP) on a multi-gram scale (>20 g) in high yield (86%). The trifunctional aromatic scaffold was then crosslinked with various diamines to demonstrate tunable viscoelastic behavior and thermal properties, with glass transition temperatures (Tg) ranging from 9 to 147 °C, degradation temperatures (5% mass loss) up to approximately 370 °C, and excellent char yields up to 68% at 650 °C under nitrogen. Moreover, the vitrimers displayed mechanical reprocessability over five destruction/healing cycles and rapid chemical recyclability following acidic hydrolysis at mild temperatures. Our findings indicate that vitrimers possessing tunable properties and high-performance thermomechanical behavior can be easily constructed from vanillin and electrophilic aromatic scaffolds for applications in heat-shielding materials and ablative coatings.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Staudinger H. Über Polymerisation. Ber. Dtsch. Chem. Ges. A/B. 1920;53:1073–1085. doi: 10.1002/cber.19200530627. doi: 10.1002/cber.19200530627. - DOI
-
- Natali M. Kenny J. M. Torre L. Science and Technology of Polymeric Ablative Materials for Thermal Protection Systems and Propulsion Devices: A Review. Prog. Mater. Sci. 2016;84:192–275. doi: 10.1016/j.pmatsci.2016.08.003. doi: 10.1016/j.pmatsci.2016.08.003. - DOI
-
- Hale R. C. King A. E. Ramirez J. M. La Guardia M. Nidel C. Durable Plastic Goods: A Source of Microplastics and Chemical Additives in the Built and Natural Environments. Environ. Sci. Technol. Lett. 2022;9:798–807. doi: 10.1021/acs.estlett.2c00417. doi: 10.1021/acs.estlett.2c00417. - DOI
-
- Millican J. M. Agarwal S. Plastic Pollution: A Material Problem? Macromolecules. 2021;54:4455–4469. doi: 10.1021/acs.macromol.0c02814. doi: 10.1021/acs.macromol.0c02814. - DOI
-
- George K. Panda B. P. Mohanty S. Nayak S. K. Recent Developments in Elastomeric Heat Shielding Materials for Solid Rocket Motor Casing Application for Future Perspective. Polym. Adv. Technol. 2018;29:8–21. doi: 10.1002/pat.4101. doi: 10.1002/pat.4101. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
