C1 esterase inhibitor-mediated immunosuppression in COVID-19: Friend or foe?
- PMID: 38013973
- PMCID: PMC9068237
- DOI: 10.1016/j.clicom.2022.05.001
C1 esterase inhibitor-mediated immunosuppression in COVID-19: Friend or foe?
Abstract
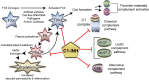
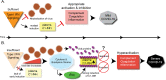
From asymptomatic to severe, SARS-CoV-2, causative agent of COVID-19, elicits varying disease severities. Moreover, understanding innate and adaptive immune responses to SARS-CoV-2 is imperative since variants such as Omicron negatively impact adaptive antibody neutralization. Severe COVID-19 is, in part, associated with aberrant activation of complement and Factor XII (FXIIa), initiator of contact system activation. Paradoxically, a protein that inhibits the three known pathways of complement activation and FXIIa, C1 esterase inhibitor (C1-INH), is increased in COVID-19 patient plasma and is associated with disease severity. Here we review the role of C1-INH in the regulation of innate and adaptive immune responses. Additionally, we contextualize regulation of C1-INH and SERPING1, the gene encoding C1-INH, by other pathogens and SARS viruses and propose that viral proteins bind to C1-INH to inhibit its function in severe COVID-19. Finally, we review the current clinical trials and published results of exogenous C1-INH treatment in COVID-19 patients.
Keywords: C1 esterase inhibitor; C1 esterase inhibitor, C1-INH; C1-INH; COVID-19; Complement; FXII; Inflammation; Middle East respiratory syndrome coronavirus, MERS-CoV; Mycobacterium tuberculosis, Mtb; Severe acute respiratory syndrome coronavirus, SARS-CoV; acquired C1-INH deficiency, AEE; activated plasma kallikrein, PKa; antibody-mediated rejection, AMR; bradykinin, BK; contact system, CS; coronavirus disease 2019, COVID-19; exogenous C1-INH, exC1-INH; hereditary angioedema, HAE; high-molecular-weight kininogen, HK; human immunodeficiency virus, HIV; interferon, IFN; interleukin, IL; ischemia/reperfusion injury, IRI; mannose-binding lectin, MBL; prekallikrein, PK; recombinant C1-INH, rhC1-INH; serine protease inhibitor, serpin; tuberculosis, TB.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- 2020. WHO Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int Accessed January 4, 2021.
-
- 2020. Coronavirus disease 2019 (COVID-19): Clinical features.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-clin... Accessed September 14, 2020.
-
- Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z., Guo L., Yang J., Wang C., Jiang S., Yang D., Zhang G., Li H., Chen F., Xu Y., Chen M., Gao Z., Yang J., Dong J., Liu B., Zhang X., Wang W., He K., Jin Q., Li M., Wang J. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe. 2020;27(6):883-890 e2. - PMC - PubMed
-
- To K.K., Hung I.F., Ip J.D., Chu A.W., Chan W.M., Tam A.R., Fong C.H., Yuan S., Tsoi H.W., Ng A.C., Lee L.L., Wan P., Tso E., To W.K., Tsang D., Chan K.H., Huang J.D., Kok K.H., Cheng V.C., Yuen K.Y. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin. Infect. Dis. 2020 - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
