This is a preprint.
A nuclear architecture screen in Drosophila identifies Stonewall as a link between chromatin position at the nuclear periphery and germline stem cell fate
- PMID: 38014085
- PMCID: PMC10680830
- DOI: 10.1101/2023.11.17.567611
A nuclear architecture screen in Drosophila identifies Stonewall as a link between chromatin position at the nuclear periphery and germline stem cell fate
Update in
-
A nuclear architecture screen in Drosophila identifies Stonewall as a link between chromatin position at the nuclear periphery and germline stem cell fate.Genes Dev. 2024 Jun 25;38(9-10):415-435. doi: 10.1101/gad.351424.123. Genes Dev. 2024. PMID: 38866555 Free PMC article.
Abstract
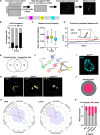
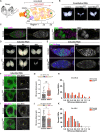
The association of genomic loci to the nuclear periphery is proposed to facilitate cell-type specific gene repression and influence cell fate decisions. However, the interplay between gene position and expression remains incompletely understood, in part because the proteins that position genomic loci at the nuclear periphery remain unidentified. Here, we used an Oligopaint-based HiDRO screen targeting ~1000 genes to discover novel regulators of nuclear architecture in Drosophila cells. We identified the heterochromatin-associated protein, Stonewall (Stwl), as a factor promoting perinuclear chromatin positioning. In female germline stem cells (GSCs), Stwl binds and positions chromatin loci, including GSC differentiation genes, at the nuclear periphery. Strikingly, Stwl-dependent perinuclear positioning is associated with transcriptional repression, highlighting a likely mechanism for Stwl's known role in GSC maintenance and ovary homeostasis. Thus, our study identifies perinuclear anchors in Drosophila and demonstrates the importance of gene repression at the nuclear periphery for cell fate.
Keywords: Genome organization; Germline stem cell; Heterochromatin; Nuclear architecture; Nuclear periphery.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
