This is a preprint.
Macrophage Depletion Protects Against Cisplatin-Induced Ototoxicity and Nephrotoxicity
- PMID: 38014097
- PMCID: PMC10680818
- DOI: 10.1101/2023.11.16.567274
Macrophage Depletion Protects Against Cisplatin-Induced Ototoxicity and Nephrotoxicity
Update in
-
Macrophage depletion protects against cisplatin-induced ototoxicity and nephrotoxicity.Sci Adv. 2024 Jul 26;10(30):eadk9878. doi: 10.1126/sciadv.adk9878. Epub 2024 Jul 24. Sci Adv. 2024. PMID: 39047106 Free PMC article.
Abstract
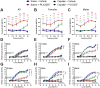
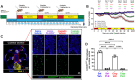
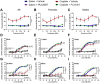
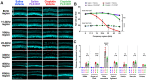
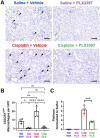
Cisplatin is a widely used and highly effective anti-cancer drug with significant side effects including ototoxicity and nephrotoxicity. Macrophages, the major resident immune cells in the cochlea and kidney, are important drivers of both inflammatory and tissue repair responses. To investigate the roles of macrophages in cisplatin-induced ototoxicity and nephrotoxicity, we used PLX3397, an FDA-approved inhibitor of the colony-stimulating factor 1 receptor (CSF1R), to eliminate tissue-resident macrophages during the course of cisplatin administration. Mice treated with cisplatin alone (cisplatin/vehicle) had significant hearing loss (ototoxicity) as well as kidney injury (nephrotoxicity). Macrophage ablation using PLX3397 resulted in significantly reduced hearing loss measured by auditory brainstem responses (ABR) and distortion-product otoacoustic emissions (DPOAE). Sensory hair cells in the cochlea were protected against cisplatin-induced death in mice treated with PLX3397. Macrophage ablation also protected against cisplatin-induced nephrotoxicity, as evidenced by markedly reduced tubular injury and fibrosis as well as reduced plasma blood urea nitrogen (BUN) and neutrophil gelatinase-associated lipocalin (NGAL) levels. Mechanistically, our data suggest that the protective effect of macrophage ablation against cisplatin-induced ototoxicity and nephrotoxicity is mediated by reduced platinum accumulation in both the inner ear and the kidney. Together our data indicate that ablation of tissue-resident macrophages represents a novel strategy for mitigating cisplatin-induced ototoxicity and nephrotoxicity.
Keywords: Ablation; Auditory Brainstem Response (ABR); CSF1R; Cisplatin; Hair cells; Kidney; Macrophages; Nephrotoxicity; Ototoxicity; PLX3397.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
