This is a preprint.
Cryptococcus neoformans rapidly invades the murine brain by sequential breaching of airway and endothelial tissues barriers, followed by engulfment by microglia
- PMID: 38014111
- PMCID: PMC10680653
- DOI: 10.1101/2023.11.13.564824
Cryptococcus neoformans rapidly invades the murine brain by sequential breaching of airway and endothelial tissues barriers, followed by engulfment by microglia
Update in
-
Cryptococcus neoformans rapidly invades the murine brain by sequential breaching of airway and endothelial tissues barriers, followed by engulfment by microglia.mBio. 2024 Apr 10;15(4):e0307823. doi: 10.1128/mbio.03078-23. Epub 2024 Mar 21. mBio. 2024. PMID: 38511961 Free PMC article.
Abstract
The fungus Cryptococcus neoformans causes lethal meningitis in humans with weakened immune systems and is estimated to account for 10-15% of AIDS-associated deaths worldwide. There are major gaps in our understanding of how this environmental fungus evades the immune system and invades the mammalian brain before the onset of overt symptoms. To investigate the dynamics of C. neoformans tissue invasion, we mapped early fungal localisation and host cell interactions at early times in infected brain, lung, and upper airways using mouse models of systemic and airway infection. To enable this, we developed an in situ imaging pipeline capable of measuring large volumes of tissue while preserving anatomical and cellular information by combining thick tissue sections, tissue clarification, and confocal imaging. Made possible by these techniques, we confirm high fungal burden in mouse upper airway turbinates after nasal inoculation. Surprisingly, most yeasts in turbinates were titan cells, indicating this microenvironment enables titan cell formation with faster kinetics than reported in mouse lungs. Importantly, we observed one instance of fungal cells enmeshed in lamina propria of upper airways, suggesting penetration of airway mucosa as a possible route of tissue invasion and dissemination to the bloodstream. We extend previous literature positing bloodstream dissemination of C. neoformans, via imaging C. neoformans within blood vessels of mouse lungs and finding viable fungi in the bloodstream of mice a few days after intranasal infection, suggesting that bloodstream access can occur via lung alveoli. In a model of systemic cryptococcosis, we show that as early as 24 h post infection, majority of C. neoformans cells traversed the blood-brain barrier, and are engulfed or in close proximity to microglia. Our work establishes that C. neoformans can breach multiple tissue barriers within the first days of infection. This work presents a new method for investigating cryptococcal invasion mechanisms and demonstrates microglia as the primary cells responding to C. neoformans invasion.
Figures



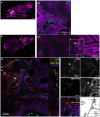

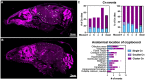
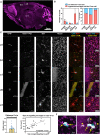

Similar articles
-
Cryptococcus neoformans rapidly invades the murine brain by sequential breaching of airway and endothelial tissues barriers, followed by engulfment by microglia.mBio. 2024 Apr 10;15(4):e0307823. doi: 10.1128/mbio.03078-23. Epub 2024 Mar 21. mBio. 2024. PMID: 38511961 Free PMC article.
-
Intranasal Inoculation of Cryptococcus neoformans in Mice Produces Nasal Infection with Rapid Brain Dissemination.mSphere. 2019 Aug 7;4(4):e00483-19. doi: 10.1128/mSphere.00483-19. mSphere. 2019. PMID: 31391283 Free PMC article.
-
Glucuronoxylomannan intranasal challenge prior to Cryptococcus neoformans pulmonary infection enhances cerebral cryptococcosis in rodents.PLoS Pathog. 2023 Apr 28;19(4):e1010941. doi: 10.1371/journal.ppat.1010941. eCollection 2023 Apr. PLoS Pathog. 2023. PMID: 37115795 Free PMC article.
-
Cryptococcus neoformans Infection in the Central Nervous System: The Battle between Host and Pathogen.J Fungi (Basel). 2022 Oct 12;8(10):1069. doi: 10.3390/jof8101069. J Fungi (Basel). 2022. PMID: 36294634 Free PMC article. Review.
-
Cryptococcus neoformans-astrocyte interactions: effect on fungal blood brain barrier disruption, brain invasion, and meningitis progression.Crit Rev Microbiol. 2021 Mar;47(2):206-223. doi: 10.1080/1040841X.2020.1869178. Epub 2021 Jan 21. Crit Rev Microbiol. 2021. PMID: 33476528 Free PMC article. Review.
References
-
- WHO fungal priority pathogens list to guide research, development and public health action. https://www.who.int/publications-detail-redirect/9789240060241.
-
- Goldman D.L., Khine H., Abadi J., Lindenberg D.J., Pirofski L., Niang R., and Casadevall A. (2001). Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107, E66. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
