This is a preprint.
Poc1 is a basal body inner junction protein that promotes triplet microtubule integrity and interconnections
- PMID: 38014135
- PMCID: PMC10680851
- DOI: 10.1101/2023.11.17.567593
Poc1 is a basal body inner junction protein that promotes triplet microtubule integrity and interconnections
Update in
-
Poc1 bridges basal body inner junctions to promote triplet microtubule integrity and connections.J Cell Biol. 2024 Aug 5;223(8):e202311104. doi: 10.1083/jcb.202311104. Epub 2024 May 14. J Cell Biol. 2024. PMID: 38743010 Free PMC article.
Abstract
Basal bodies (BBs) are conserved eukaryotic structures that organize motile and primary cilia. The BB is comprised of nine, cylindrically arranged, triplet microtubules (TMTs) that are connected to each other by inter-TMT linkages which maintain BB structure. During ciliary beating, forces transmitted to the BB must be resisted to prevent BB disassembly. Poc1 is a conserved BB protein important for BBs to resist ciliary forces. To understand how Poc1 confers BB stability, we identified the precise position of Poc1 binding in the Tetrahymena BB and the effect of Poc1 loss on BB structure. Poc1 binds at the TMT inner junctions, stabilizing TMTs directly. From this location, Poc1 also stabilizes inter-TMT linkages throughout the BB, including the cartwheel pinhead and the inner scaffold. Moreover, we identify a molecular response to ciliary forces via a molecular remodeling of the inner scaffold, as determined by differences in Fam161A localization. Thus, while not essential for BB assembly, Poc1 promotes BB interconnections that establish an architecture competent to resist ciliary forces.
Keywords: basal body; cilia; inner junction; microtubule.
Figures


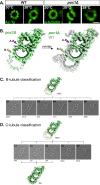


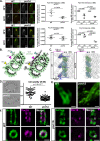
Similar articles
-
Poc1 bridges basal body inner junctions to promote triplet microtubule integrity and connections.J Cell Biol. 2024 Aug 5;223(8):e202311104. doi: 10.1083/jcb.202311104. Epub 2024 May 14. J Cell Biol. 2024. PMID: 38743010 Free PMC article.
-
Tetrahymena Poc1 ensures proper intertriplet microtubule linkages to maintain basal body integrity.Mol Biol Cell. 2016 Aug 1;27(15):2394-403. doi: 10.1091/mbc.E16-03-0165. Epub 2016 Jun 1. Mol Biol Cell. 2016. PMID: 27251062 Free PMC article.
-
Basal bodies bend in response to ciliary forces.Mol Biol Cell. 2022 Dec 1;33(14):ar146. doi: 10.1091/mbc.E22-10-0468-T. Epub 2022 Oct 26. Mol Biol Cell. 2022. PMID: 36287828 Free PMC article.
-
Tetrahymena basal bodies.Cilia. 2016 Jan 19;5:1. doi: 10.1186/s13630-016-0022-8. eCollection 2015. Cilia. 2016. PMID: 26793300 Free PMC article. Review.
-
Ciliate cortical organization and dynamics for cell motility: Comparing ciliates and vertebrates.J Eukaryot Microbiol. 2022 Sep;69(5):e12880. doi: 10.1111/jeu.12880. Epub 2022 Jan 12. J Eukaryot Microbiol. 2022. PMID: 34897878 Free PMC article. Review.
References
-
- Beck B.B., Phillips J.B., Bartram M.P., Wegner J., Thoenes M., Pannes A., Sampson J., Heller R., Göbel H., Koerber F., Neugebauer A., Hedergott A., Nürnberg G., Nürnberg P., Thiele H., Altmüller J., Toliat M.R., Staubach S., Boycott K.M., Valente E.M., Janecke A.R., Eisenberger T., Bergmann C., Tebbe L., Wang Y., Wu Y., Fry A.M., Westerfield M., Wolfrum U., and Bolz H.J.. 2014. Mutation of POC1B in a severe syndromic retinal ciliopathy. Hum Mutat. 35:1153–1162. doi:10.1002/humu.22618. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
