This is a preprint.
CXCL9 recombinant adeno-associated virus (AAV) virotherapy sensitizes glioblastoma (GBM) to anti-PD-1 immune checkpoint blockade
- PMID: 38014191
- PMCID: PMC10680939
- DOI: 10.21203/rs.3.rs-3463730/v1
CXCL9 recombinant adeno-associated virus (AAV) virotherapy sensitizes glioblastoma (GBM) to anti-PD-1 immune checkpoint blockade
Update in
-
Adeno-associated virus delivered CXCL9 sensitizes glioblastoma to anti-PD-1 immune checkpoint blockade.Nat Commun. 2024 Jul 12;15(1):5871. doi: 10.1038/s41467-024-49989-1. Nat Commun. 2024. PMID: 38997283 Free PMC article.
Abstract
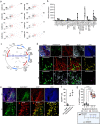
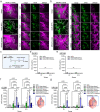
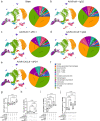
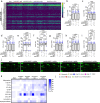
The promise of immunotherapy to induce long-term durable responses in conventionally treatment resistant tumors like glioblastoma (GBM) has given hope for patients with a dismal prognosis. Yet, few patients have demonstrated a significant survival benefit despite multiple clinical trials designed to invigorate immune recognition and tumor eradication. Insights gathered over the last two decades have revealed numerous mechanisms by which glioma cells resist conventional therapy and evade immunological detection, underscoring the need for strategic combinatorial treatments as necessary to achieve appreciable therapeutic effects. However, new combination therapies are inherently difficult to develop as a result of dose-limiting toxicities, the constraints of the blood-brain barrier, and the suppressive nature of the GBM tumor microenvironment (TME). GBM is notoriously devoid of lymphocytes driven in part by a paucity of lymphocyte trafficking factors necessary to prompt their recruitment, infiltration, and activation. We have developed a novel recombinant adeno-associated virus (AAV) gene therapy strategy that enables focal and stable reconstitution of the GBM TME with C-X-C motif ligand 9 (CXCL9), a powerful call-and-receive chemokine for cytotoxic T lymphocytes (CTLs). By precisely manipulating local chemokine directional guidance, AAV-CXCL9 increases tumor infiltration by CD8-postive cytotoxic lymphocytes, sensitizing GBM to anti-PD-1 immune checkpoint blockade (ICB). These effects are accompanied by immunologic signatures evocative of an inflamed and responsive TME. These findings support targeted AAV gene therapy as a promising adjuvant strategy for reconditioning GBM immunogenicity given its excellent safety profile, TME-tropism, modularity, and off-the-shelf capability, where focal delivery bypasses the constrains of the blood-brain barrier, further mitigating risks observed with high-dose systemic therapy.
Conflict of interest statement
Declarations Conflict of Interest The authors declare no potential conflicts of interest
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

