This is a preprint.
VTA dopamine neurons are hyperexcitable in 3xTg-AD mice due to casein kinase 2-dependent SK channel dysfunction
- PMID: 38014232
- PMCID: PMC10680865
- DOI: 10.1101/2023.11.16.567486
VTA dopamine neurons are hyperexcitable in 3xTg-AD mice due to casein kinase 2-dependent SK channel dysfunction
Update in
-
VTA dopamine neurons are hyperexcitable in 3xTg-AD mice due to casein kinase 2-dependent SK channel dysfunction.Nat Commun. 2024 Nov 8;15(1):9673. doi: 10.1038/s41467-024-53891-1. Nat Commun. 2024. PMID: 39516200 Free PMC article.
Abstract
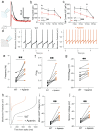
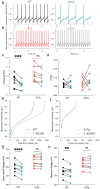
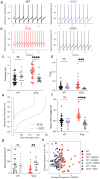
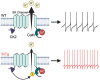
Alzheimer's disease (AD) patients exhibit neuropsychiatric symptoms that extend beyond classical cognitive deficits, suggesting involvement of subcortical areas. Here, we investigated the role of midbrain dopamine (DA) neurons in AD using the amyloid + tau-driven 3xTg-AD mouse model. We found deficits in reward-based operant learning in AD mice, suggesting possible VTA DA neuron dysregulation. Physiological assessment revealed hyperexcitability and disrupted firing in DA neurons caused by reduced activity of small-conductance calcium-activated potassium (SK) channels. RNA sequencing from contents of single patch-clamped DA neurons (Patch-seq) identified up-regulation of the SK channel modulator casein kinase 2 (CK2). Pharmacological inhibition of CK2 restored SK channel activity and normal firing patterns in 3xTg-AD mice. These findings shed light on a complex interplay between neuropsychiatric symptoms and subcortical circuits in AD, paving the way for novel treatment strategies.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
