This is a preprint.
Dorsal Motor Vagal Neurons Can Elicit Bradycardia and Reduce Anxiety-Like Behavior
- PMID: 38014247
- PMCID: PMC10680764
- DOI: 10.1101/2023.11.14.566855
Dorsal Motor Vagal Neurons Can Elicit Bradycardia and Reduce Anxiety-Like Behavior
Update in
-
Dorsal motor vagal neurons can elicit bradycardia and reduce anxiety-like behavior.iScience. 2024 Feb 6;27(3):109137. doi: 10.1016/j.isci.2024.109137. eCollection 2024 Mar 15. iScience. 2024. PMID: 38420585 Free PMC article.
Abstract
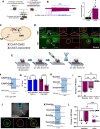
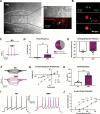
Cardiovagal neurons (CVNs) innervate cardiac ganglia through the vagus nerve to control cardiac function. Although the cardioinhibitory role of CVNs in nucleus ambiguus (CVNNA) is well established, the nature and functionality of CVNs in dorsal motor nucleus of the vagus (CVNDMV) is less clear. We therefore aimed to characterize CVNDMV anatomically, physiologically, and functionally. Optogenetically activating cholinergic DMV neurons resulted in robust bradycardia through peripheral muscarinic (parasympathetic) and nicotinic (ganglionic) acetylcholine receptors, but not beta-1-adrenergic (sympathetic) receptors. Retrograde tracing from the cardiac fat pad labeled CVNNA and CVNDMV through the vagus nerve. Using whole cell patch clamp, CVNDMV demonstrated greater hyperexcitability and spontaneous action potential firing ex vivo despite similar resting membrane potentials, compared to CVNNA. Chemogenetically activating DMV also caused significant bradycardia with a correlated reduction in anxiety-like behavior. Thus, DMV contains uniquely hyperexcitable CVNs capable of cardioinhibition and robust anxiolysis.
Keywords: autonomic regulation; parasympathetic nervous system.
Conflict of interest statement
DECLARATION OF INTEREST Authors declare no competing interest.
Figures


Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
