This is a preprint.
Reversible histone deacetylase activity catalyzes lysine acylation
- PMID: 38014285
- PMCID: PMC10680841
- DOI: 10.1101/2023.11.17.567549
Reversible histone deacetylase activity catalyzes lysine acylation
Update in
-
Reversible histone deacetylase activity catalyzes lysine acylation.Nat Chem Biol. 2025 Sep;21(9):1387-1396. doi: 10.1038/s41589-025-01869-5. Epub 2025 Mar 26. Nat Chem Biol. 2025. PMID: 40140626
Abstract
Starvation and low carbohydrate diets lead to the accumulation of the ketone body, β-hydroxybutyrate (BHB), whose blood concentrations increase more than 10-fold into the millimolar range. In addition to providing a carbon source, BHB accumulation triggers lysine β-hydroxybutyrylation (Kbhb) of proteins via unknown mechanisms. As with other lysine acylation events, Kbhb marks can be removed by histone deacetylases (HDACs). Here, we report that class I HDACs unexpectedly catalyze protein lysine modification with β-hydroxybutyrate (BHB). Mutational analyses of the HDAC2 active site reveal a shared reliance on key amino acids for classical deacetylation and non-canonical HDAC-catalyzed β-hydroxybutyrylation. Also consistent with reverse HDAC activity, Kbhb formation is driven by mass action and substrate availability. This reverse HDAC activity is not limited to BHB but also extends to multiple short-chain fatty acids. The reversible activity of class I HDACs described here represents a novel mechanism of PTM deposition relevant to metabolically-sensitive proteome modifications.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



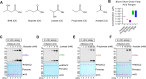
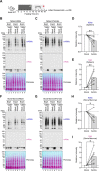
References
-
- Taggart A. K. et al. , (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem 280, 26649–26652 (2005). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
