This is a preprint.
Insulin at the Intersection of Thermoregulation and Glucose Homeostasis
- PMID: 38014310
- PMCID: PMC10680846
- DOI: 10.1101/2023.11.17.566254
Insulin at the Intersection of Thermoregulation and Glucose Homeostasis
Update in
-
Insulin at the intersection of thermoregulation and glucose homeostasis.Mol Metab. 2024 Mar;81:101901. doi: 10.1016/j.molmet.2024.101901. Epub 2024 Feb 13. Mol Metab. 2024. PMID: 38354854 Free PMC article.
Abstract
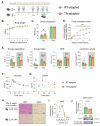
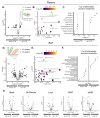
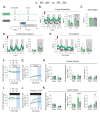
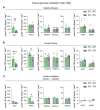
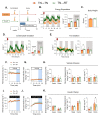
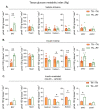
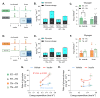
Mammals are protected from changes in environmental temperature by altering energetic processes that modify heat production. Insulin is the dominant stimulus of glucose uptake and metabolism, which are fundamental for thermogenic processes. The purpose of this work was to determine the interaction of ambient temperature induced changes in energy expenditure (EE) on the insulin sensitivity of glucose fluxes. Short-term and adaptive responses to thermoneutral temperature (TN, ~28°C) and room (laboratory) temperature (RT, ~22°C) were studied in mice. This range of temperature does not cause detectable changes in circulating catecholamines or shivering and postabsorptive glucose homeostasis is maintained. We tested the hypothesis that a decrease in EE that occurs with TN causes insulin resistance and that this reduction in insulin action and EE is reversed upon short term (<12h) transition to RT. Insulin-stimulated glucose disposal (Rd) and tissue specific glucose uptake were assessed combining isotopic tracers with hyperinsulinemic-euglycemic clamps. EE and insulin-stimulated Rd are both decreased (~50%) in TN-adapted vs RT-adapted mice. When RT-adapted mice are switched to TN, EE rapidly decreases and Rd is reduced by ~50%. TN-adapted mice switched to RT exhibit a rapid increase in EE, but whole body insulin-stimulated Rd remains at the low rates of TN-adapted mice. In contrast, whole body glycolytic flux rose with EE. This higher EE occurs without increasing glucose uptake from the blood, but rather by diverting glucose from glucose storage to glycolysis. In addition to adaptations in insulin action, 'insulin-independent' glucose uptake in brown fat is exquisitely sensitive to thermoregulation. These results show that insulin action adjusts to non-stressful changes in ambient temperature to contribute to the support of body temperature homeostasis without compromising glucose homeostasis.
Figures
Similar articles
-
Insulin at the intersection of thermoregulation and glucose homeostasis.Mol Metab. 2024 Mar;81:101901. doi: 10.1016/j.molmet.2024.101901. Epub 2024 Feb 13. Mol Metab. 2024. PMID: 38354854 Free PMC article.
-
Coupling of energy intake and energy expenditure across a temperature spectrum: impact of diet-induced obesity in mice.Am J Physiol Endocrinol Metab. 2020 Sep 1;319(3):E472-E484. doi: 10.1152/ajpendo.00041.2020. Epub 2020 Jul 21. Am J Physiol Endocrinol Metab. 2020. PMID: 32691631 Free PMC article.
-
Short-term thermoneutral housing alters glucose metabolism and markers of adipose tissue browning in response to a high-fat diet in lean mice.Am J Physiol Regul Integr Comp Physiol. 2018 Oct 1;315(4):R627-R637. doi: 10.1152/ajpregu.00364.2017. Epub 2018 May 23. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 29791203 Free PMC article.
-
Energy and substrate metabolism in obesity and postobese state.Diabetes Care. 1991 Dec;14(12):1180-8. doi: 10.2337/diacare.14.12.1180. Diabetes Care. 1991. PMID: 1773703 Review.
-
Thermoregulation in endothermic insects.Science. 1974 Aug 30;185(4153):747-56. doi: 10.1126/science.185.4153.747. Science. 1974. PMID: 4602075 Review.
References
-
- Castellini M. Encyclopedia of marine mammals. Elsevier; 2009:1166–71.
-
- Camastra S, Bonora E, Del Prato S, Rett K, Weck M, and Ferrannini E. Effect of obesity and insulin resistance on resting and glucose-induced thermogenesis in man. EGIR (European Group for the Study of Insulin Resistance). Int J Obes Relat Metab Disord. 1999;23(12):1307–13. - PubMed
-
- Ravussin E, and Bogardus C. Thermogenic response to insulin and glucose infusions in man: a model to evaluate the different components of the thermic effect of carbohydrate. Life Sci. 1982;31(18):2011–8. - PubMed
-
- Golay A, DeFronzo RA, Ferrannini E, Simonson DC, Thorin D, Acheson K, et al. Oxidative and non-oxidative glucose metabolism in non-obese type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1988;31(8):585–91. - PubMed
-
- Yokoyama H, Mori K, Emoto M, Araki T, Teramura M, Mochizuki K, et al. Non-oxidative glucose disposal is reduced in type 2 diabetes, but can be restored by aerobic exercise. Diabetes Obes Metab. 2008;10(5):400–7. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
