This is a preprint.
Engineered odorant receptors illuminate structural principles of odor discrimination
- PMID: 38014344
- PMCID: PMC10680712
- DOI: 10.1101/2023.11.16.567230
Engineered odorant receptors illuminate structural principles of odor discrimination
Update in
-
Engineered odorant receptors illuminate the basis of odour discrimination.Nature. 2024 Nov;635(8038):499-508. doi: 10.1038/s41586-024-08126-0. Epub 2024 Oct 30. Nature. 2024. PMID: 39478229
Abstract
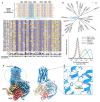
A central challenge in olfaction is understanding how the olfactory system detects and distinguishes odorants with diverse physicochemical properties and molecular configurations. Vertebrate animals perceive odors via G protein-coupled odorant receptors (ORs). In humans, ~400 ORs enable the sense of smell. The OR family is composed of two major classes: Class I ORs are tuned to carboxylic acids while Class II ORs, representing the vast majority of the human repertoire, respond to a wide variety of odorants. How ORs recognize chemically diverse odorants remains poorly understood. A fundamental bottleneck is the inability to visualize odorant binding to ORs. Here, we uncover fundamental molecular properties of odorant-OR interactions by employing engineered ORs crafted using a consensus protein design strategy. Because such consensus ORs (consORs) are derived from the 17 major subfamilies of human ORs, they provide a template for modeling individual native ORs with high sequence and structural homology. The biochemical tractability of consORs enabled four cryoEM structures of distinct consORs with unique ligand recognition properties. The structure of a Class I consOR, consOR51, showed high structural similarity to the native human receptor OR51E2 and yielded a homology model of a related member of the human OR51 family with high predictive power. Structures of three Class II consORs revealed distinct modes of odorant-binding and activation mechanisms between Class I and Class II ORs. Thus, the structures of consORs lay the groundwork for understanding molecular recognition of odorants by the OR superfamily.
Conflict of interest statement
H.M. has received royalties from Chemcom, research grants from Givaudan, and consultant fees from Kao. A.M. is a founder of Epiodyne and Stipple Bio, consults for Abalone, and serves on the scientific advisory board of Septerna.
Figures





References
-
- Bjarnadóttir T. K. et al. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics 88, 263–273 (2006). - PubMed
-
- Glusman G., Yanai I., Rubin I. & Lancet D. The complete human olfactory subgenome. Genome Res. 11, 685–702 (2001). - PubMed
-
- Buck L. & Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65, 175–187 (1991). - PubMed
-
- Liberles S. D. & Buck L. B. A second class of chemosensory receptors in the olfactory epithelium. Nature 442, 645–650 (2006). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
