Usefulness of the Rapid Antigen Test in Detecting SARS-CoV-2 for Infection Control in Hospitals
- PMID: 38014727
- PMCID: PMC10990885
- DOI: 10.3947/ic.2023.0077
Usefulness of the Rapid Antigen Test in Detecting SARS-CoV-2 for Infection Control in Hospitals
Abstract
Background: We aimed at evaluating the diagnostic performance of rapid antigen test (RAT) compared to polymerase chain reaction (PCR) for severe acute respiratory syndrome coronavirus 2 and the possible transmission of infection to close contacts from patients with negative RAT and positive PCR results.
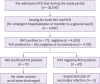
Materials and methods: Patients/guardians urgently requiring admission to the ward on the same day had been hospitalized with RAT-negative result before the PCR results were available. We performed an epidemiologic investigation of the close contacts of those with negative RAT but positive PCR results after hospitalization.
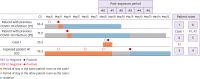
Results: A total of 4,237 RATs were performed from March to August 2022. When the PCR test was used as the reference, RAT had a sensitivity of 28.8% (17/59; 95% confidence interval [CI], 17.8 - 42.1), a specificity of 100% (4,220/4,220; 95% CI, 99.9 - 100.0), a positive predictive value of 100.0% (17/17; 95% CI, 100.0 - 100.0), and a negative predictive value of 99.0% (4,178/4,220; 95% CI, 99.3 - 99.8). The epidemiologic investigation revealed that among the 32 patients with negative RAT and subsequent positive PCR results after admission into multi-patient room, two (6.3%) showed secondary coronavirus disease 2019.
Conclusion: The secondary transmission rate from patients with negative RAT and positive PCR results was low. Our data suggest that RAT may be useful for rapid exclusion of high transmissible cases. However, further evaluation using whole genome sequencing is needed to determine the potential for transmissibility in cases showing a negative RAT but a positive PCR result.
Keywords: COVID-19; Contact tracing; Infection control; Rapid diagnostic test.
Copyright © 2024 by The Korean Society of Infectious Diseases, Korean Society for Antimicrobial Therapy, and The Korean Society for AIDS.
Conflict of interest statement
No conflict of interest.
Figures

Comment in
-
Response to Usefulness of the Rapid Antigen Test in Detecting SARS-CoV-2 for Infection Control in Hospitals.Infect Chemother. 2024 Jun;56(2):282-283. doi: 10.3947/ic.2024.0035. Epub 2024 May 13. Infect Chemother. 2024. PMID: 38859719 Free PMC article. No abstract available.
Similar articles
-
[Evaluation of the Rapid Antigen Detection Kit with the Polymerase Chain Reaction for Detection of SARS-CoV-2 in Respiratory Samples].Mikrobiyol Bul. 2022 Apr;56(2):263-273. doi: 10.5578/mb.20229806. Mikrobiyol Bul. 2022. PMID: 35477229 Turkish.
-
Universal screening for SARS-CoV-2 infection: a rapid review.Cochrane Database Syst Rev. 2020 Sep 15;9(9):CD013718. doi: 10.1002/14651858.CD013718. Cochrane Database Syst Rev. 2020. PMID: 33502003 Free PMC article.
-
Epidemiologic Characteristics Associated With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antigen-Based Test Results, Real-Time Reverse Transcription Polymerase Chain Reaction (rRT-PCR) Cycle Threshold Values, Subgenomic RNA, and Viral Culture Results From University Testing.Clin Infect Dis. 2021 Sep 15;73(6):e1348-e1355. doi: 10.1093/cid/ciab303. Clin Infect Dis. 2021. PMID: 33846714 Free PMC article.
-
Diagnostic performance of rapid antigen tests (RAT) for COVID-19 and factors associated with RAT-negative results among RT-PCR-positive individuals during Omicron BA.2, BA.5 and XBB.1 predominance.BMC Infect Dis. 2024 May 21;24(1):504. doi: 10.1186/s12879-024-09408-8. BMC Infect Dis. 2024. PMID: 38769524 Free PMC article.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Sep 30;9:CD013639. doi: 10.1002/14651858.CD013639.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. PMID: 32997361 Updated.
Cited by
-
Response to Usefulness of the Rapid Antigen Test in Detecting SARS-CoV-2 for Infection Control in Hospitals.Infect Chemother. 2024 Jun;56(2):282-283. doi: 10.3947/ic.2024.0035. Epub 2024 May 13. Infect Chemother. 2024. PMID: 38859719 Free PMC article. No abstract available.
References
-
- Centers for Disease Control and Prevention (CDC) Overview of testing for SARS-CoV-2, the virus that causes COVID-19. [Accessed 4 September, 2023]. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html.
-
- Yamayoshi S, Sakai-Tagawa Y, Koga M, Akasaka O, Nakachi I, Koh H, Maeda K, Adachi E, Saito M, Nagai H, Ikeuchi K, Ogura T, Baba R, Fujita K, Fukui T, Ito F, Hattori SI, Yamamoto K, Nakamoto T, Furusawa Y, Yasuhara A, Ujie M, Yamada S, Ito M, Mitsuya H, Omagari N, Yotsuyanagi H, Iwatsuki-Horimoto K, Imai M, Kawaoka Y. Comparison of rapid antigen tests for COVID-19. Viruses. 2020;12:1420. - PMC - PubMed
-
- Scheiblauer H, Filomena A, Nitsche A, Puyskens A, Corman VM, Drosten C, Zwirglmaier K, Lange C, Emmerich P, Müller M, Knauer O, Nübling CM. Comparative sensitivity evaluation for 122 CE-marked rapid diagnostic tests for SARS-CoV-2 antigen, Germany, September 2020 to April 2021. Euro Surveill. 2021;26:2100441. - PMC - PubMed
-
- The R Foundation. The R project for statistical computing. [Accessed 31 October 2022]. Available at: https://www.R-project.org/
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

