Pharmacokinetics and Tolerability of the Cancer-Targeting MRI Contrast Agent MT218 in Healthy Males
- PMID: 38015107
- PMCID: PMC10987082
- DOI: 10.1097/RLI.0000000000001031
Pharmacokinetics and Tolerability of the Cancer-Targeting MRI Contrast Agent MT218 in Healthy Males
Abstract
Objective: The aim of this study was to evaluate the pharmacokinetics and safety profile of MT218, a peptide-targeted gadolinium-based contrast agent, in healthy males.
Materials and methods: This was a double-blind, randomized, placebo-controlled, single-ascending-dose study including 30 healthy male subjects. In each dose group (0.01, 0.02, 0.04, and 0.08 mmol/kg), 4 subjects received MT218 and 2 subjects received placebo (saline) in bolus injections. The highest dose group (0.08 mmol/kg) was assessed in 2 cohorts, 1 fasted and 1 nonfasted. Clinical laboratory tests, vital signs, and electrocardiograms were investigated. Gadolinium concentrations were measured in plasma samples collected before administration and over a 24-hour period postinjection, and in urine specimens collected until 22 days. A noncompartmental model was used for pharmacokinetic analysis. A clinical and biological safety follow-up was carried out for up to 6 months.
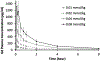
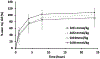
Results: No clinically significant modifications in biochemistry, hematology, urinalysis, electrocardiogram parameters, or vital signs were reported at any time point for any treatment group. No serious adverse events were observed in any dose group. Transient dizziness, hyperhidrosis, and injection site coldness were the main adverse events reported in both the MT218 and placebo groups. The mean total apparent clearance decreased slightly with increasing dose, and the median plasma t 1/2 ranged from 1.7 hours in the 0.01 mmol/kg group to 2.7 hours in the 0.08 mmol/kg nonfasted group. MT218 was rapidly excreted via renal filtration with 42.9% to 52.8% of the injected dose measured in urine within the first hour after administration, and 92.5% to 117.3% in urine within 24 hours. No Gd was detected by inductively coupled plasma mass spectrometry in urine after 21 days.
Conclusion: Single intravenous administration of MT218 was safely tolerated in the healthy males. Its pharmacokinetic parameters and safety profile are well aligned with those of other gadolinium-based contrast agents.
Copyright © 2023 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest none declared.
Figures
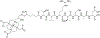
Similar articles
-
Pharmacokinetics, Safety, and Tolerability of the Novel Tetrameric, High-Relaxivity, Macrocyclic Gadolinium-Based Contrast Agent Gadoquatrane in Healthy Adults.Invest Radiol. 2024 Feb 1;59(2):140-149. doi: 10.1097/RLI.0000000000001043. Epub 2023 Nov 3. Invest Radiol. 2024. PMID: 37921759 Free PMC article. Clinical Trial.
-
Detection of Brain Metastases by Contrast-Enhanced MRI: Comparison of Gadopiclenol and Gadobenate in a Mouse Model.Invest Radiol. 2024 Feb 1;59(2):131-139. doi: 10.1097/RLI.0000000000001032. Epub 2023 Nov 3. Invest Radiol. 2024. PMID: 37921777 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Safety, tolerability, pharmacokinetics, and pharmacodynamics of the first-in-class GLP-1 and amylin receptor agonist, amycretin: a first-in-human, phase 1, double-blind, randomised, placebo-controlled trial.Lancet. 2025 Jul 12;406(10499):135-148. doi: 10.1016/S0140-6736(25)01176-6. Epub 2025 Jun 20. Lancet. 2025. PMID: 40550229 Clinical Trial.
-
Single-dose intravenous diclofenac for acute postoperative pain in adults.Cochrane Database Syst Rev. 2018 Aug 28;8(8):CD012498. doi: 10.1002/14651858.CD012498.pub2. Cochrane Database Syst Rev. 2018. PMID: 30153336 Free PMC article.
Cited by
-
MR Molecular Imaging of Extradomain-B Fibronectin for Assessing Progression and Therapy Resistance of Prostate Cancer.Chem Biomed Imaging. 2024 Jun 11;2(8):560-568. doi: 10.1021/cbmi.4c00002. eCollection 2024 Aug 26. Chem Biomed Imaging. 2024. PMID: 39211789 Free PMC article.
-
Assessment of the Efficacy of the Combination of RNAi of lncRNA DANCR with Chemotherapy to Treat Triple Negative Breast Cancer Using Magnetic Resonance Molecular Imaging.Bioconjug Chem. 2024 Mar 20;35(3):381-388. doi: 10.1021/acs.bioconjchem.4c00001. Epub 2024 Mar 6. Bioconjug Chem. 2024. PMID: 38446033 Free PMC article.
References
-
- Lu ZR, Laney V, and Li Y, Targeted Contrast Agents for Magnetic Resonance Molecular Imaging of Cancer. Acc Chem Res, 2022. 55(19): p. 2833–2847. - PubMed
-
- Nunn AD, Linder KE, and Tweedle MF, Can receptors be imaged with MRI agents? Q J Nucl Med, 1997. 41(2): p. 155–62. - PubMed
-
- Artemov D., et al., MR molecular imaging of the Her-2/neu receptor in breast cancer cells using targeted iron oxide nanoparticles. Magn Reson Med, 2003. 49(3): p. 403–8. - PubMed
-
- Sipkins DA, et al., Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat Med, 1998. 4(5): p. 623–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

